
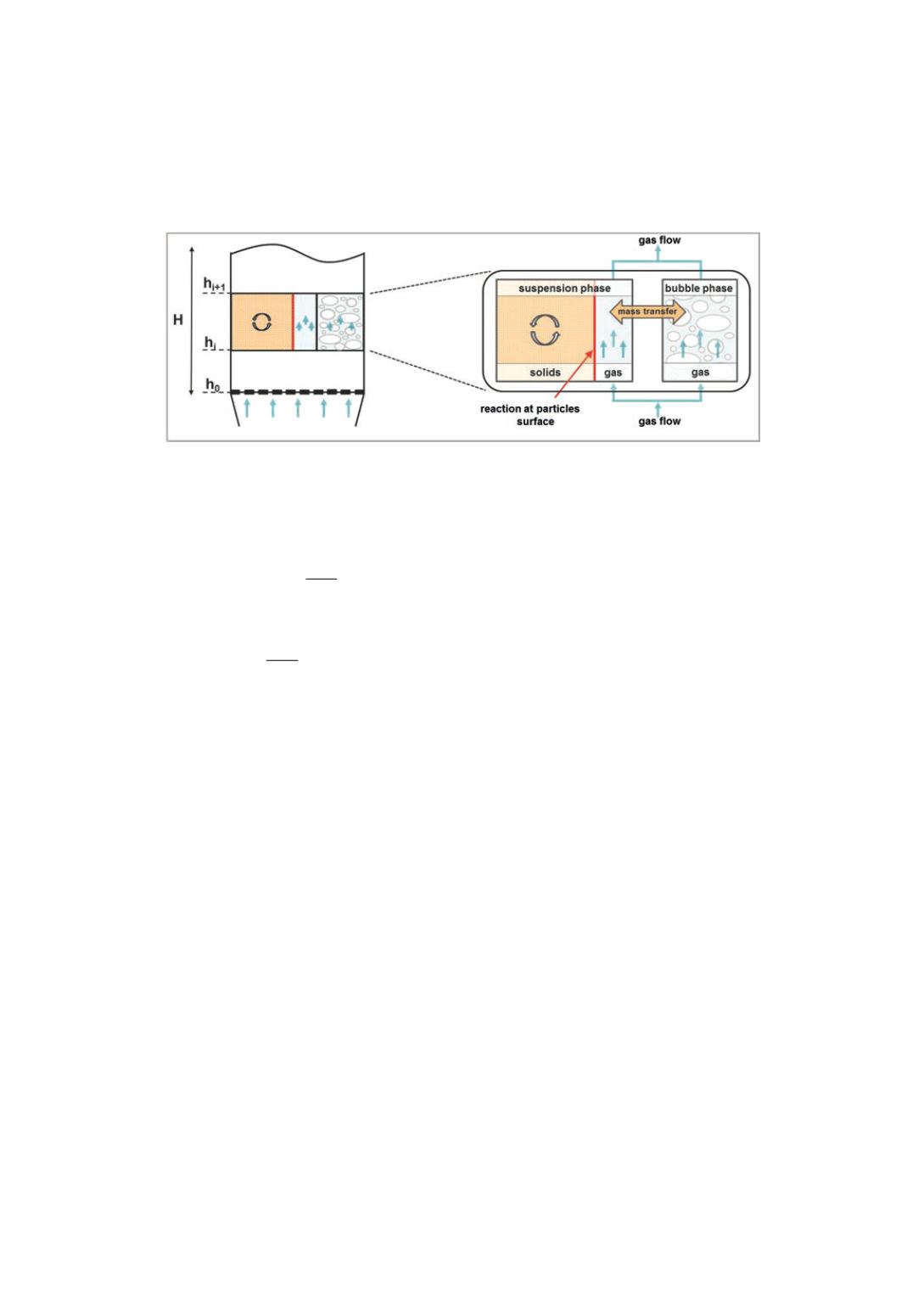
is accessible for the chemical reaction only by mass transport from the “bubble phase”
into the “suspension phase”. The driving force considered for the mass transport is the
concentration gradient of the respective components.
Figure 4
: Schematic of the two phase model approach used
The differential gas mass balances for the two phases are formulated as follows:
Bubble phase:
(
)
[
]
(
)
is
ib b iG
ib
b
mf
c ca k
h
c
uu
,
,
,
,
1
− ⋅
−=
∂
∂
− ⋅
−
ε
(2)
Suspension phase:
(
)
(
)
(
)
educt
ir P mf
b
is
ib b iG
is
b
mf
c k a
c ca k
h
c
u
⋅
⋅
⋅
⋅
−+ − ⋅
−=
∂
∂
−
,
,
,
,
,
1
1
ε
ε
ε
(3)
Where
u
denotes the superficial gas velocity, and
u
mf
and
İ
mf
are the minimum
fluidization velocity and the bed porosity at minimum fluidization respectively. It is
recommended to determine both of these parameters experimentally to characterize the
powder that is used. Alternatively
u
mf
and
İ
mf
can also be calculated from the equations
according to Ergun [10] or Wen and Yu [11] for example.
İ
b
is the local bubble volume
fraction and
c
b,i
the corresponding concentration of each component
i
within the bubble
phase. The mass transport between the two phases caused by the concentration
difference in the “bubble phase” (
c
b,i
) and the “suspension phase” (
c
s,i
) is calculated by
means of a mass transfer coefficient
k
G,i
, based on a correlation by Sit and Grace [17].
The specific mass transfer area
a
b
is determined by the overall, local gas-bubble surface
areas of each volume element. On the assumption that the heterogeneously catalyzed
gas-solid reaction only occurs within the “suspension phase,” the chemical reaction is
considered by means of a reaction constant approach, where
k
r,i
represents the reaction
constant relating to the product component
i
,
a
P
is the total specific particle surface area
that contributes to the reaction and
c
educt
refers to the availability of the gaseous reactant
(chloromethane).
To describe the ideally mixed solid phase an overall mass balance is formulated
according to Equation 4 considering the solids inlet flow rate
ۦ
in
, the particles that are
entrained with the gas flow at the reactor’s outlet
ۦ
entr
, the loss of solids due to chemical
reaction,
ۦ
reac
, as well as the particle flow rate that is separated out by the cyclones and
recycled to the reactor,
ۦ
recycle
.
161


















