
techniques with alcohols [21] or silanes like hexamethyldisilazane have been employed
[22]. Silanol group densities in the range of ca. 2 – 4.5 silanol/nm
2
have been reported
for pyrogenic silica. One factor which hampers the accurate determination of silanol
numbers is the highly reactive nature of the silica surface, analytical results are easily
biased by strong coordination of water molecules to silanols. In order to completely
remove adsorbed water, elevated temperature and reduced pressure is needed, which
can finally result in silanol condensation yielding strained siloxane bridges at the silica
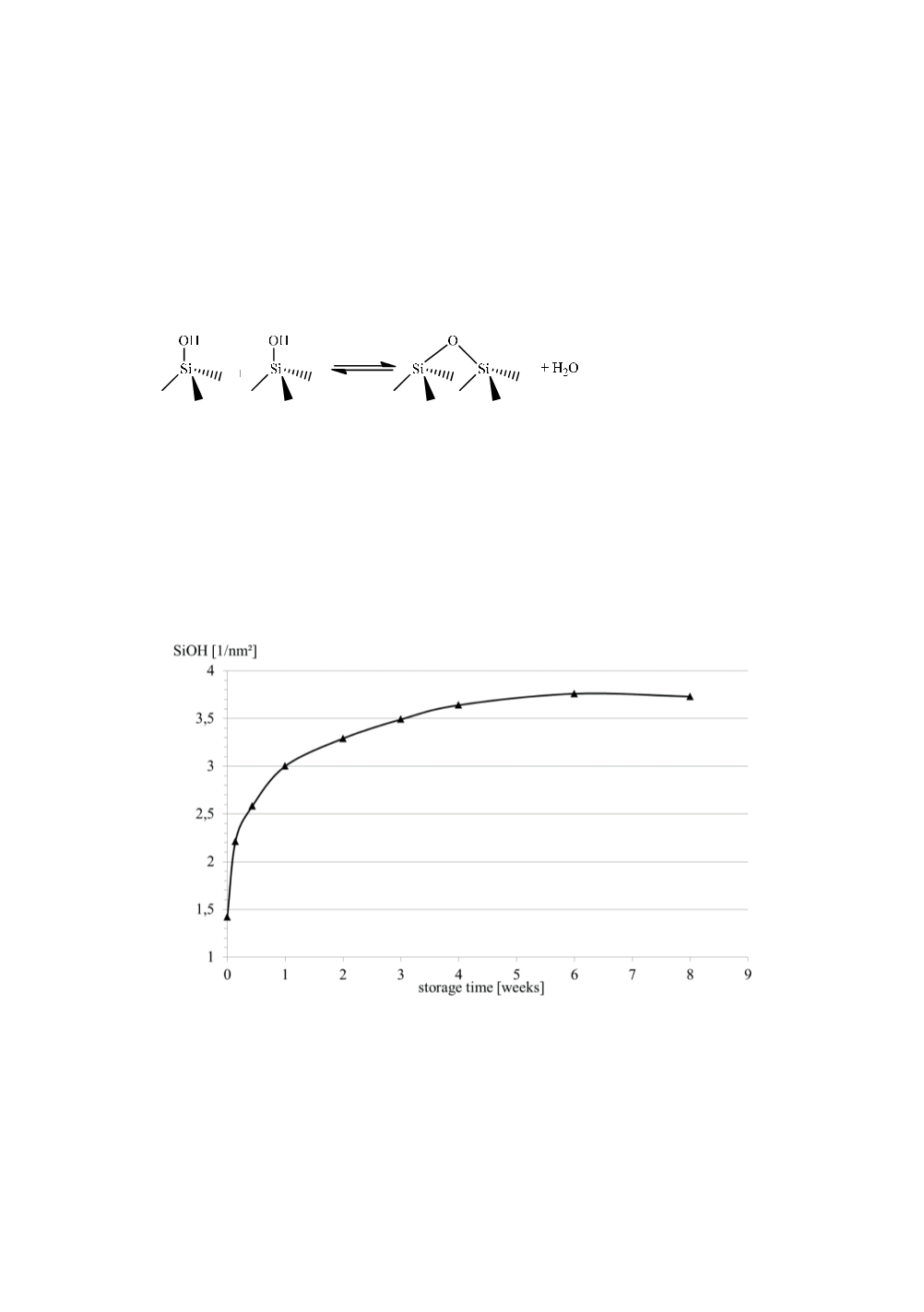
surface according to eq. 5 [23].
(5)
Formation of strained siloxane bridges from silanol condensation also has been
postulated to occur during the high-temperature production process [24]. As a
consequence, pyrogenic silica isolated immediately after the flame reactor should
reveal a low silanol group density and high reactivity toward water according to reverse
reaction of eq. 5. This is corroborated by the observations that pyrogenic silica collected
directly after the HCl desorption unit and immediately stored under dry argon exhibits
a silanol group density of only 1.3 – 1.4 silanol/nm
2
and undergoes a rapid increase of
silanol group density when stored at 94 % rel. humidity. The development of silanol
numbers over time is shown below in fig. 8 were determined by means of LiAlH
4
titration of samples that have been dynamically dried in a stream of dry nitrogen.
Figure 8:
Evolution of silanol group density of pyrogenic silica stored at 94 % rel.
humidity.
The rapid increase in silanol groups levels with time and approaches an upper limit of
ca. 3.8 SiOH/nm
2
. The same behavior was found independently in systematic studies
of silica aging under controlled humidity conditions [25, 26].
113


















