
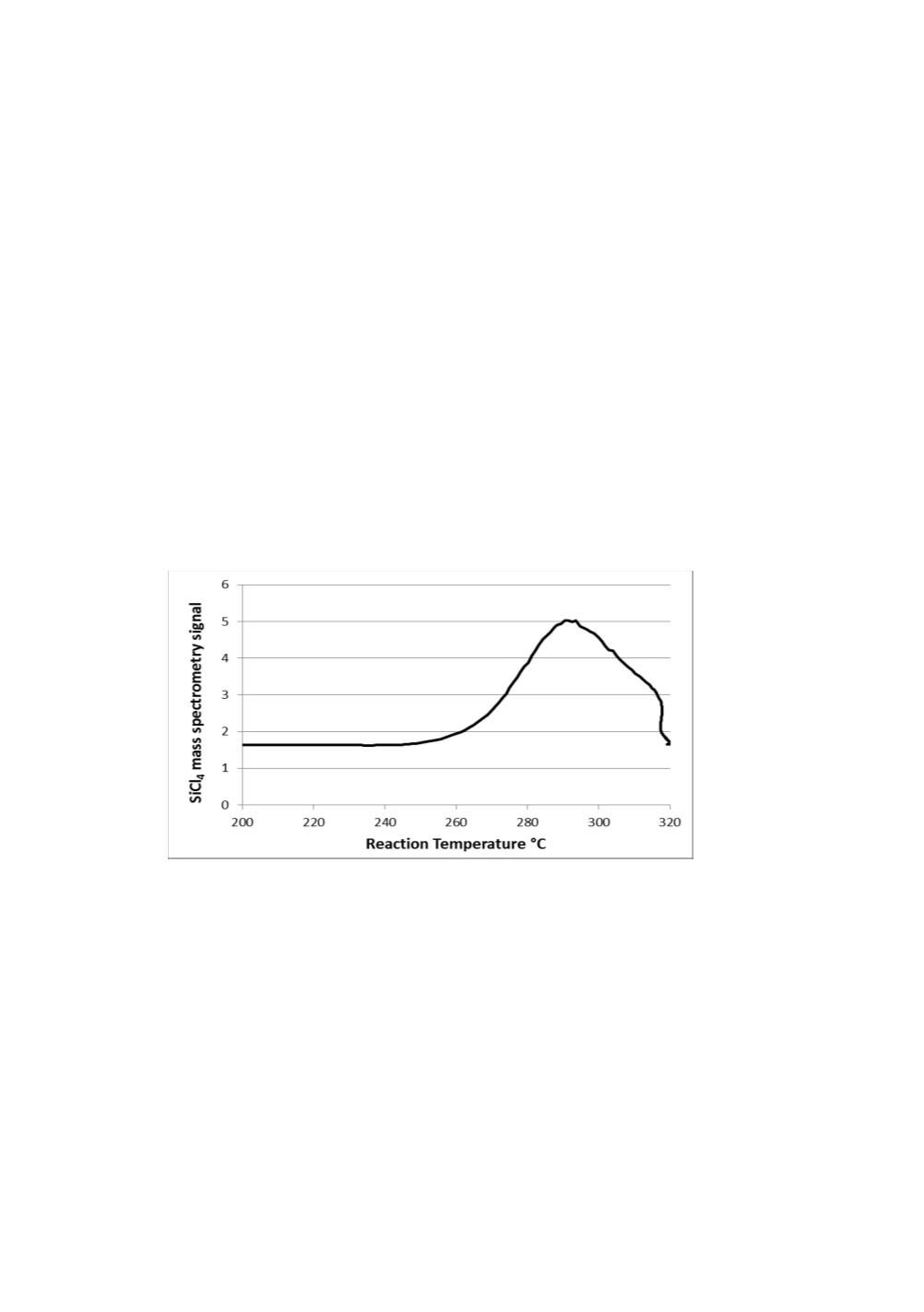
Figure 3 shows the SiCl
4
signal intensity vs reactor temperature in a CA-MS
experiment. The onset temperature for SiCl
4
formation is estimated as ~ 250 °C. This
is close to what observed in earlier studies with a differential scanning calorimetric
(DSC) method.
22
Upon completion of the reaction within similar timescale as the GC
measurement, the MS signal dropped back to baseline level. Although not as good as
the GC method for quantifying different products, the MS signal intensity is still a
quantitative indicator for SiCl
4
formation-rate change (MS signal shows linear
response to concentration change of a specific compound within a certain range,
although the responses could be very different for different compounds of the same
concentration). This reaction has excess Si and the reaction rate should be first-order
to CuCl concentration (liming reagent). Based on pseudo-first-order theory, SiCl
4
formation rate (MS intensity) is proportional to CuCl concentration.
20
Therefore, the
CA-MS method is also applicable for studying reaction kinetics.
Please note that the method here is limited to detecting SiCl
4
formation and reduction
of CuCl by Si (reaction 10). It does not measure the reaction temperature for copper
silicide formation (reaction 11), which may or may not occur at higher temperature as
a separate step. Previous study with a DSC method did not suggest a different reaction
temperature for silicide formation.
22
Figure 3.
SiCl
4
mass spectrometry signal vs reaction temperature curve.
The results from this study conform that SiCl
4
is the major product with close to
100% yield in the reaction of Si with CuCl (based on reaction 10). The methods
employed here are sensitive and suitable for studying reaction with very high Si/Cu
ratio. Preference for Si/Cu molar ratio higher than 40 in the direct process has been
reported.
23
Such high Si-Cu ratio could impose challenges for the DCS method
commonly employed in previous studies. The heat generated from the reaction of Si
with small amount of CuCl could become insignificant compared the heat required for
increasing the temperature of the reaction mass. On the other hand, earlier kinetic
studies involved hydrolysis of SiCl
4
and titration of the resulting HCl to measure
CuCl conversion.
20,21
The CA-MS method reported here should provide higher
sensitivity and significant shorter cycle time for measuring CuCl conversion, and
enable comprehensive studies on the reaction kinetics.
154


















