
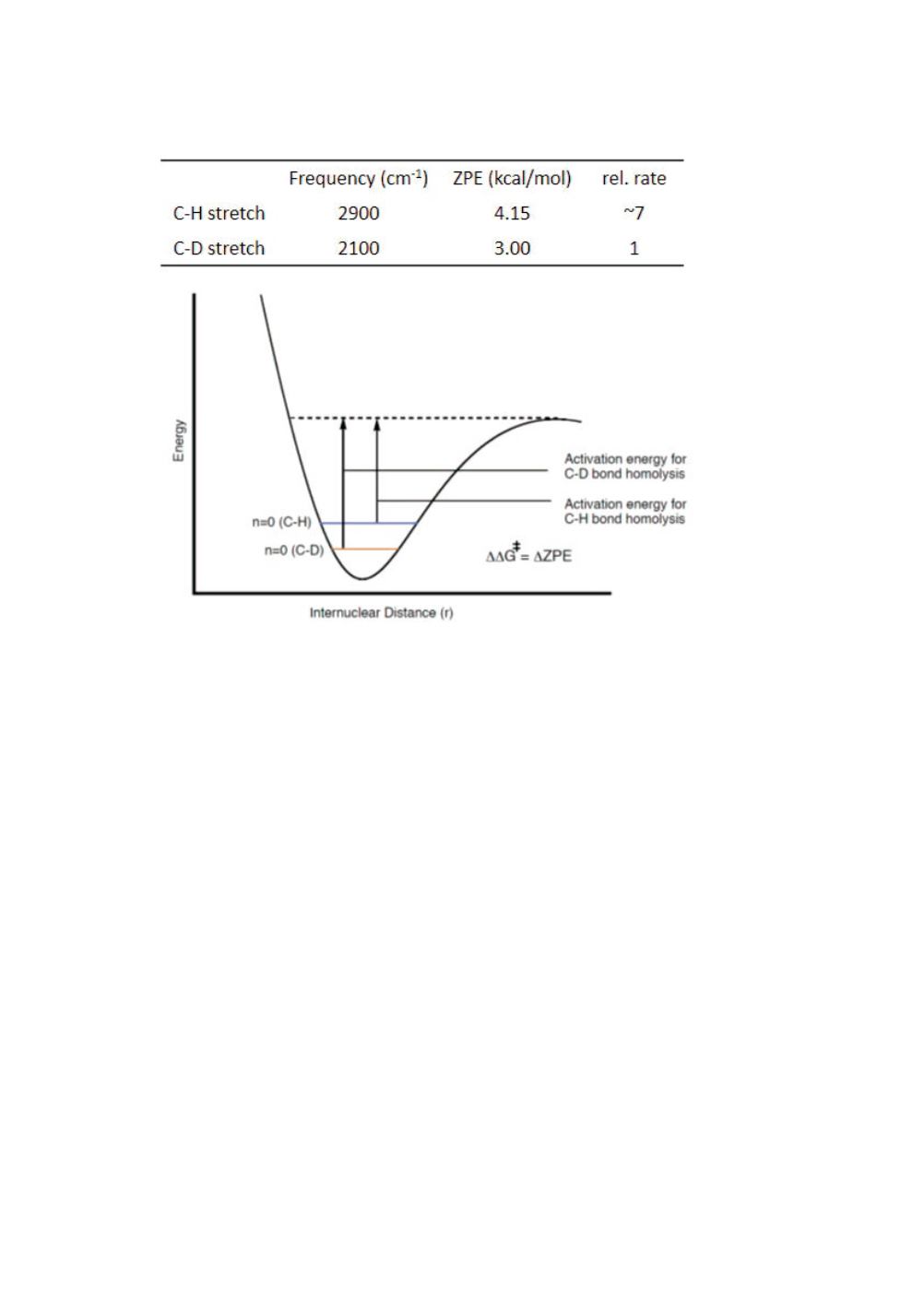
Scheme 2.
Illustration of zero point energy (ZPE) and homolytic cleavage activation
energy (
ǻ
G‡) differences for C-H and C-D bonds. Adopted from an online
publication
(https://www.princeton.edu/chemistry/macmillan/group-meetings/RRK-KIE.pdf) with modifications. The values for frequency, ZPA and relative rate are
from ref. 17.
Alber studied the direct process with deuterated methyl chloride (CD
3
Cl).
13
In that
study, the direct process reaction was initiated with CH
3
Cl. The feed gas was then
switched back-and-forth between CD
3
Cl and CH
3
Cl. It was observed that switching to
CD
3
Cl drastically decreased production rate and selectivity of MeH, confirming that
its formation involves C-H bond cleavage as the RDS. On the other hand, Me2
production rate and selectivity increased when CD
3
Cl was fed. The increase of Me2
was comparable to the loss of MeH.
13
This observation strongly suggests that the
hydrogen ligands for Si-H formation are generated from the methyl groups which can
also be incorporated into Me2. Therefore, the hydrogen ligand in MeH should be
generated on active sites that also form chlorosilane products. Transferring methyl
groups into Si to form Me2 and cleaving C-H bond to form MeH are two competing
reactions.
On the other hand, feeding CD
3
Cl reduced methane formation but increased ethane
formation.
13
This observation confirms that C-H bond cleavage is also a key
component of the RDS in methane formation. Moreover, methane formation through
reaction 3 is competing with ethane formation through combination of two methyl
groups.
151


















