
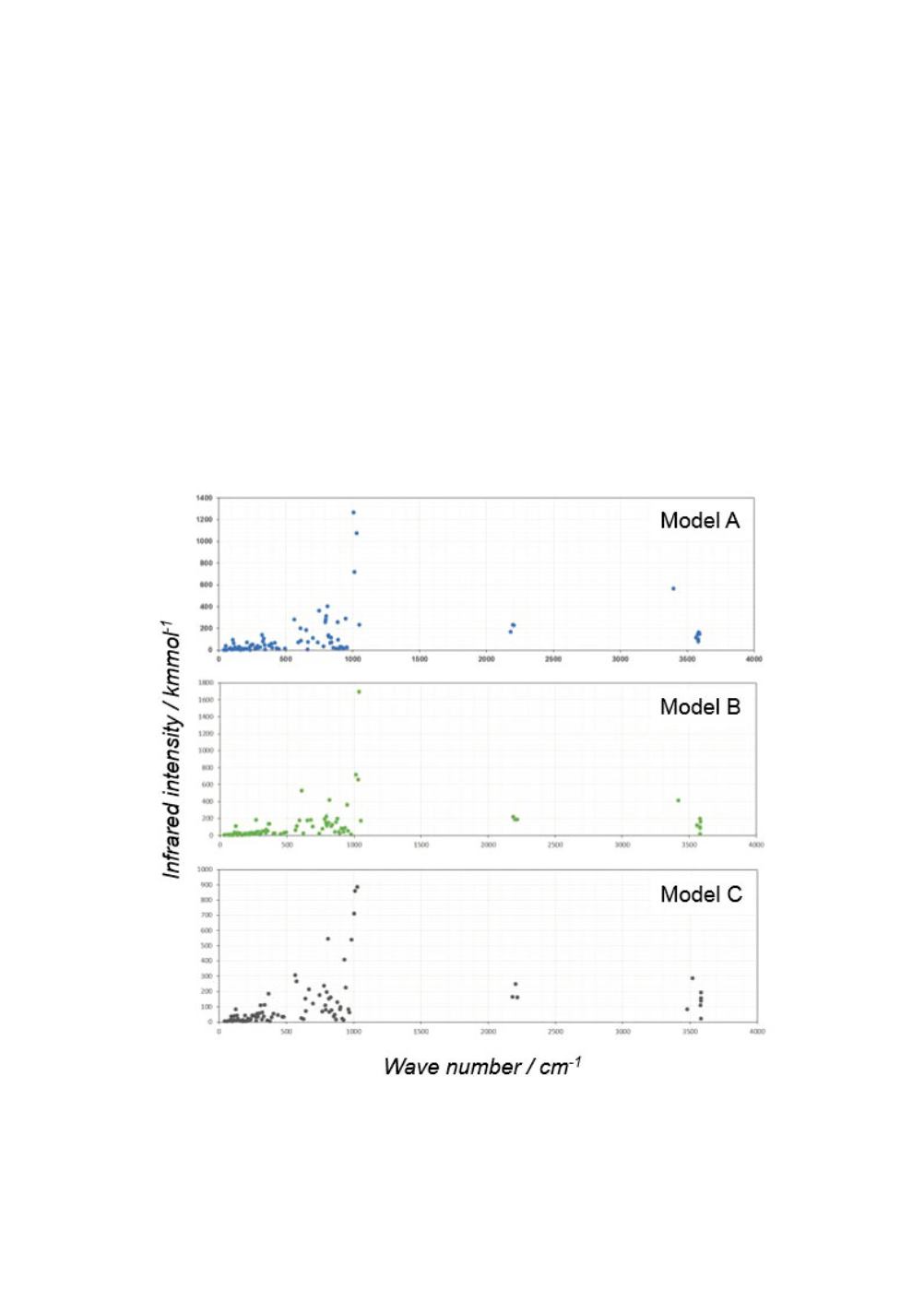
The molar absorptivity or infrared intensity was also calculated for each vibrational
mode.
The scaled molecular vibrational spectra are shown for Models A ~ C with partial
hexahedral cage in Figure 8, and Models D ~ F with twisted cage with one diagonal Si-
O-Si bond in Figure 9.
Seven modes of O-H stretching vibration are observed above 3000 cm
-1
for the models
of A ~ F. One of those seven modes for each of Models A and B indicates significantly
lower wave number which is affected by the hydrogen bonding. The influence of
hydrogen bond on the O-H vibration is reported by Chojnowski et al. [13] According
to them the vibrational frequency or the wave number is lowered in the range of 100 ~
400 cm
-1
depending on the type of hydrogen bond complex.
There are three modes of Si-H stretching vibration in 2000 ~ 2500 cm
-1
range common
for the spectra of Models A ~ F.
The strong infrared intensities around 1000 cm
-1
are related to Si-O vibrations.
It is difficult to describe the other infrared absorption peaks in the fingerprint region by
particular motion of component atoms. It should be noted that a peak around 875 cm
-1
was consistently observed in FTIR spectra of the actual explosive substances.
Figure 8
: Scaled molecular vibrational spectra for Models A ~ C with partial hexahedral cage
including four Si-Si bonds.
195


















