
Figure 4
: Optimized structures of two isomers of Model A with different arrangement of OH/H
on the ends of open ring. The optimization level is MP2/6-31G*.
The obtained total electronic energies plus nuclear repulsive energies are compared in
Figure 5 for three combinations of OH/OH, H/OH and H/H on the end of the open ring
of Model A.
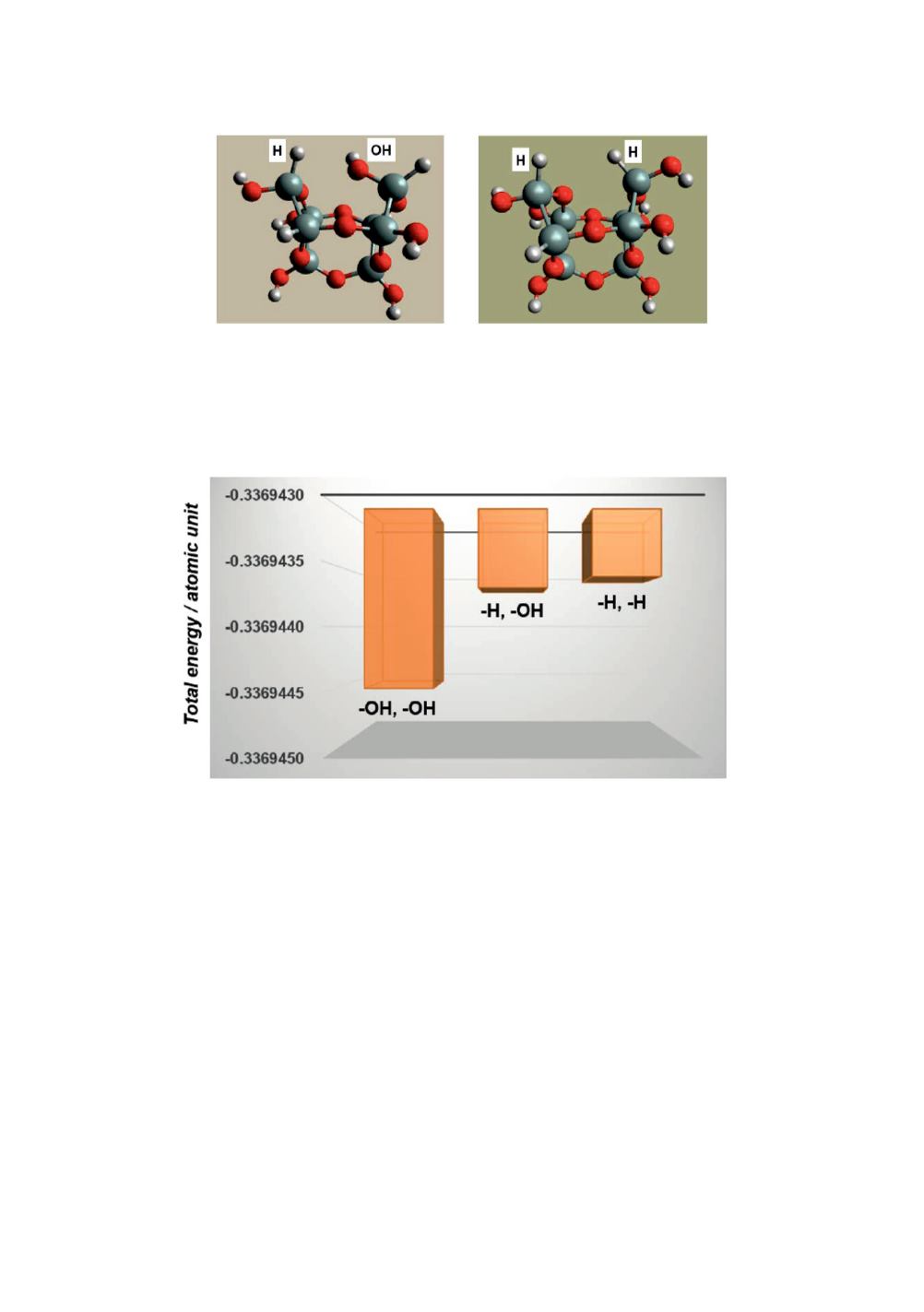
Figure 5
: Impact of H/OH replacement on the end of Model A open ring on the total electronic
energy plus nuclear repulsive energies.
As Figure 5 shows, the combination of two OH groups on the ends of the open ring
gives the lowest energy among the three isomers. This notable stabilization is attained
by hydrogen bonding between the two OH groups facing each other across the open
ring as indicated by the dashed line for Model A in Figure 2. In comparison the
difference is significantly small between the other two combinations of H/OH and H/H.
Number of Si-Si bonds per molecule
The impact of the number of Si-Si bonds on total electronic energy plus nuclear
repulsive energies was examined. Structural models based on the partial cage were
prepared for three Si-Si bonds and two S-Si bonds. They were optimized again by the
same method. The results of the optimization are given in Figure 6. The hydrogen
bond is formed between the two OH groups on the end of the open ring for both three
and two S-Si bond models as indicated by the dashed line. The position of the hydrogen
bond in those isomers is configurationally equivalent to the one in Model A.
The model of three Si-Si bonds, a stereoisomer of the models with four Si-Si bonds, is
named Model G for the further study.
193


















