
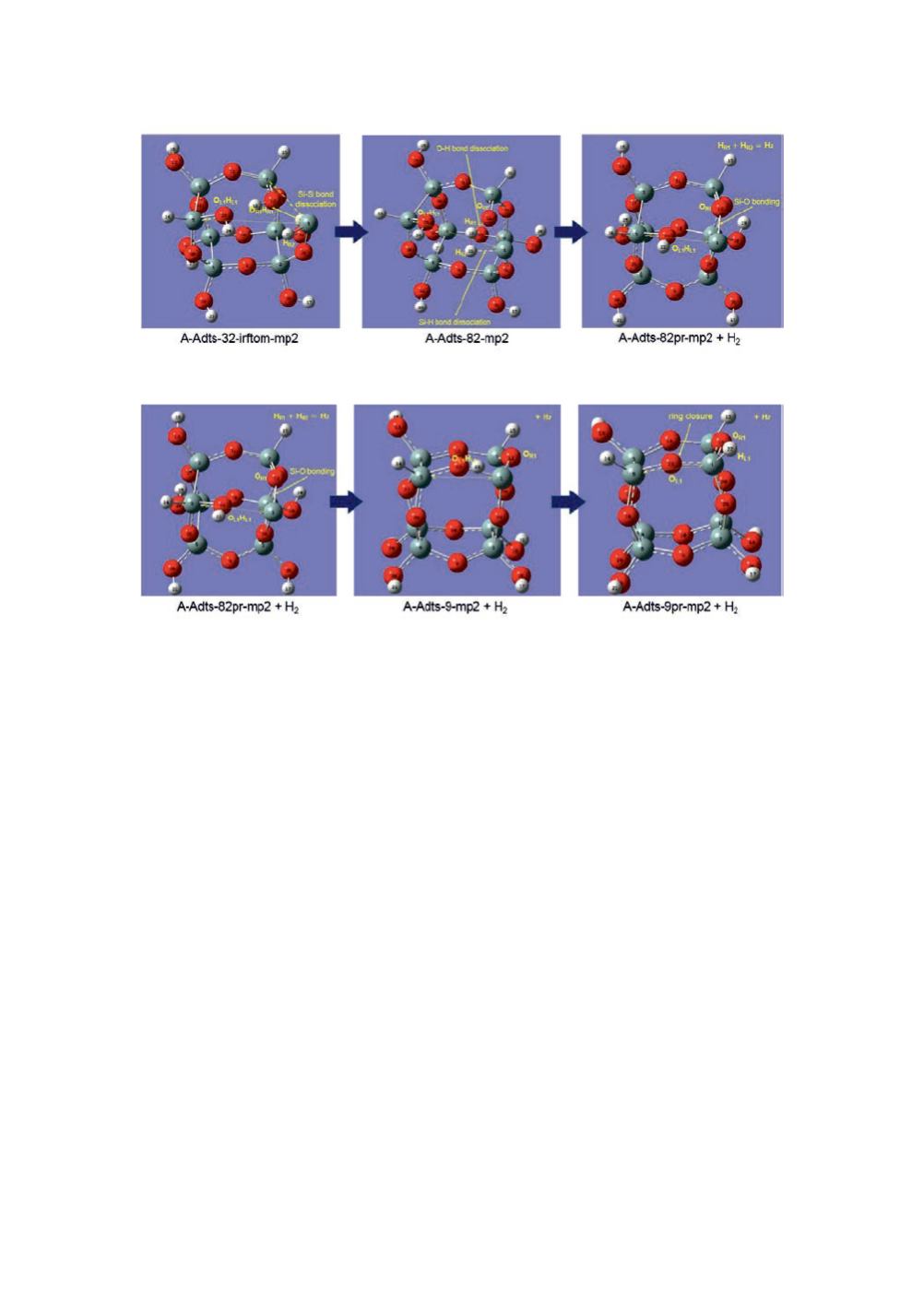
Figure 14
: Elimination of a H
2
molecule via the transition state “A-Adts-82-mp2”.
Figure 15
: 1,2-transfer of H atom and ring closure via the transition state “A-Adts-9-mp2”.
Conclusion
The ab initio molecular orbital calculations were successfully applied to the modeling
of the explosive molecule, which was based on the available information obtained in
the investigation of the cause of the accident and found in the literature.
The optimized structure of the model is described as a partial hexahedral cage
composed of four Si-Si bonds, seven Si-O-Si bonds and one open ring. Four Si-Si
bonds are schematically in parallel configuration. Three H atoms and seven OH groups
are attached to eight Si atoms on each corner of the hexahedron. There is a hydrogen
bond in the area of the open ring.
A possible intramolecular reaction path was found. The reaction path is composed of
three serial steps with three transition states and two metastable states in between. The
reaction is significantly exothermic and includes oxidation of a Si-Si bond and
formation of a H
2
molecule, which is qualitatively consistent with the observation. The
reaction may be interpreted as the initial ignition stage of the explosion.
The intermolecular reaction path for the internal oxidation will be explored in the next
step. Finally we will consider the explosion mechanism on the basis of the results
obtained so far and in the next step.
Acknowledgements
The authors would like to express their special thanks to Mr. Rikito Sato, Yokkaichi
Plant, Mitsubishi Materials Corporation, for his invaluable input and persistent support.
199


















