
and isolated each other so that there is no interaction between them. A larger negative
value, therefore, indicates that the electrons and atomic nuclei are more stabilized by
mutual interaction including formation of chemical bond.
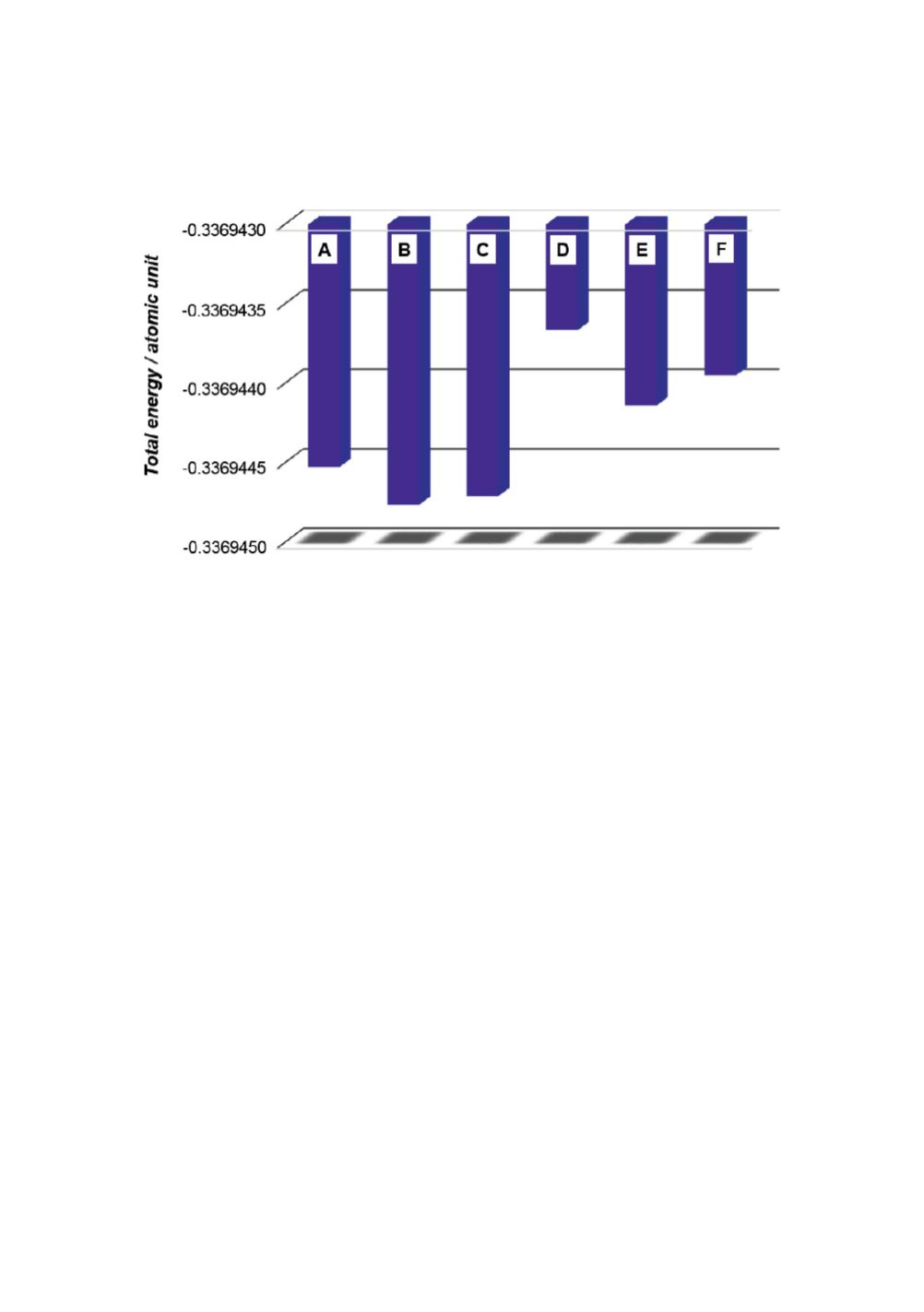
Figure 3
: Total electronic energy plus nuclear repulsive energies between the six models.
The partial cage structure of Models A ~ C is apparently more stable than the twisted
㻌
cage of Model D ~ F. The schematically parallel configuration of four Si-Si bonds of
Model A is less stable than the other two orthogonal configurations of Models B and C
in the partial cage group as illustrated in Figure 1. The similar relationship is found
between Model D and Models E and F in the twisted cage group. Model B is the most
stable among the six models and the other models are metastable in the common
potential energy surface.
They may be interpreted in terms of internal structural strain. The structural flexibility
of Si-O-Si bond more or less relaxes the strain and allows the theoretical existence of
all those stereoisomers.
H/OH replacement on the ends of open ring
Two OH groups which terminate both ends (Si atoms) of the open ring of Model A can
be replaced one by one with H atom which is bound to the other Si atom. These
replacements are expected to affect stability of the models, and therefore the structures
of those isomers of Model A were optimized by the same method described in the
previous section and the total electronic energies plus nuclear repulsive energies were
evaluated.
The structures optimized at MP2/6-31G* level are depicted in Figure 4.
192


















