
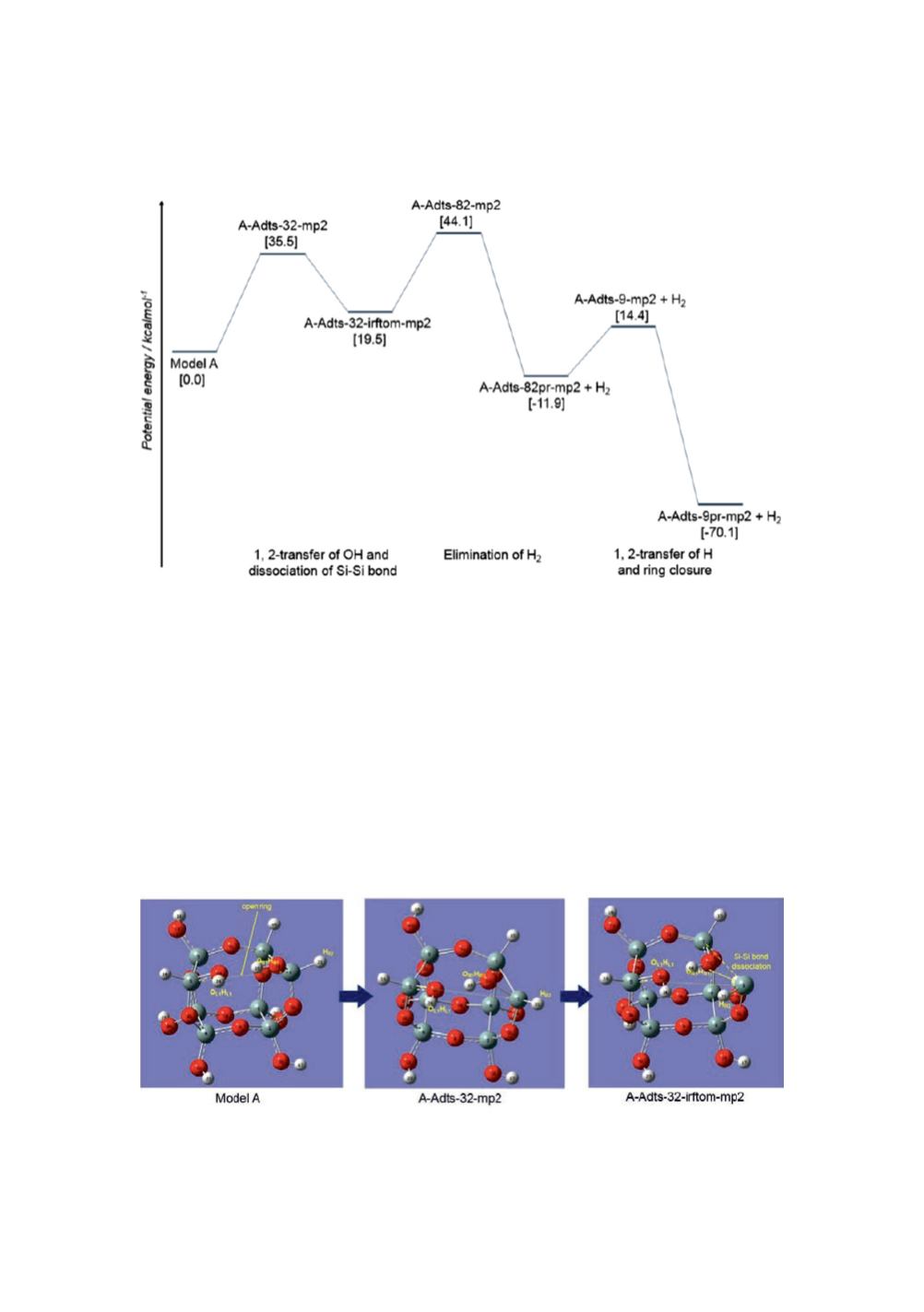
The first step starting from Model A molecule includes 1,2-transfer of OH group and
dissociation of Si-Si bond. Elimination of a H
2
molecule occurs in the second step. The
third and the last step consists of 1,2-transfer of H atom and closure of the open ring.
Figure 12
: Schematic presentation of the potential energy surface for the intramolecular
oxidation process in Model A calculated at the MP2/6-31G* level. The potential energy values
given in [ ] are corrected for zero-point energy.
The three steps or elementary reactions are illustrated in Figures 13, 14 and 15
respectively.
The overall chemical reaction is represented as
Si
8
H
10
O
14
Æ
Si
8
H
8
O
14
+ H
2
(1)
The number of Si-Si bonds per molecule decreases from four to three according to the
reaction, which is found to be significantly exothermic.
This is possibly the initial ignition stage or the trigger of the explosion since the overall
explosion reaction is thought to be still more exothermic.
Figure 13
: 1,2-transfer of OH group and dissociation of Si-Si bond the via the transition state
“A-Adts-32-mp2”.
198


















