
It must be noted that fast pyrolysis is different from the pyrolysis process applied in this
work and is used to maximize the yield and quality of the condensate. Hence, the
obtained pyrolysis oil in this work is expected to have somewhat lower quality than that
of the fast pyrolysis oils. This is also reflected on the heating value of 15.3 MJ/kg,
which is lower compared to about 17 MJ/kg reported for fast pyrolysis oils. However,
when calculated back on dry basis the heating values are on par with each other at
around 22.8 MJ/kg. The pH for the condensate is similar as for fast pyrolysis oil at 2.5
but the total acid number (TAN) is almost twice as high at 205 mg KOH/g. Hence, from
a combustion point of view, the condensate can be regarded as a "high" moisture
content pyrolysis oil. From an upgrading point of view (to chemicals or engine fuels),
larger challenges can be expected mainly due to the high TAN and high oxygen content.
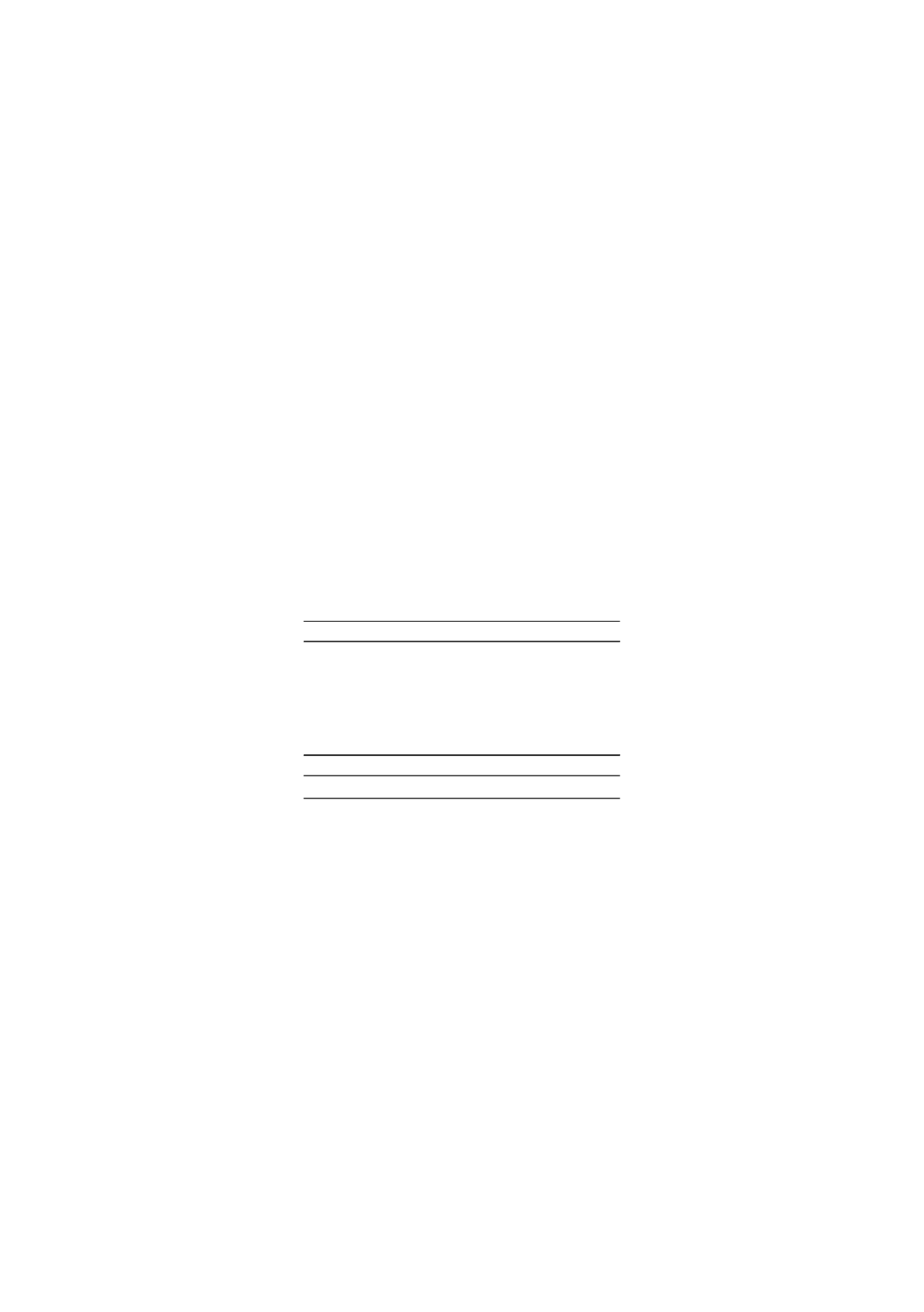
In Table 2 the mass and chemical energy balance for the experiment is showed together
with the elemental balance. The overall mass yield have been normalized whilst the
others are showed as measured and calculated, hence they do not sum to unity and the
difference can be interpreted is a reflection of the experimental uncertainty. The largest
uncertainty here lays with the oxygen balance at +10.8%. Three different methods have
been used to determine the composition of the different fractions and there is also an
inherent uncertainty whilst weighing the different samples. However, current efforts
are focused towards reducing the experimental uncertainties.
Table 2.
The overall mass and chemical energy balance including the balance for the major
elements C, H and O. Notice that the amount of gas in the overall mass balance was calculated
by difference.
On mass basis; 25.7% of the dry biomass is converted into charcoal with the
composition showed in Table 1. However, close to 45% of the chemical energy is found
in the charcoal, around 55% is found in the condensate and the remaining 4.4% in the
gas. It should be emphasized that in a larger scale pyrolyser less condensate and more
gas is expected since the pyrolysis vapours can be given longer residence time.
Typically longer vapour residence time results in secondary reactions which again
results in a small increase in charcoal production but a significant increase in gas
production and a consequential decrease in condensate production. However, since the
condensate may have a larger outside-gate value then the inside-gate value of the gas
this needs to be taken into account when different full scale pyrolysis technologies are
considered. The carbon content of the biomass feedstock is more or less equally
distributed between the charcoal and the condensate with a remainder of 6.9 % found
in the gas. For hydrogen and oxygen the condensate dominates; claiming 85% and 90%
of the available atoms, respectively.
Charcoal Condensate Gas Sum
Yield
25.7
64.3 10.0 100
C
42.7
42.8 6.9 92.4
H
11.2
85.3 3.1 99.6
O
5.4
90.1 15.3 110.8
Yield
47.0
55.4 4.4 106.8
a) gas calculted by differnce
Mass balance
(
wt-%
)
E
ner
gy
balance
(
%
)
a
5


















