
Department of Chemical Engineering
Annual Report 2015
26
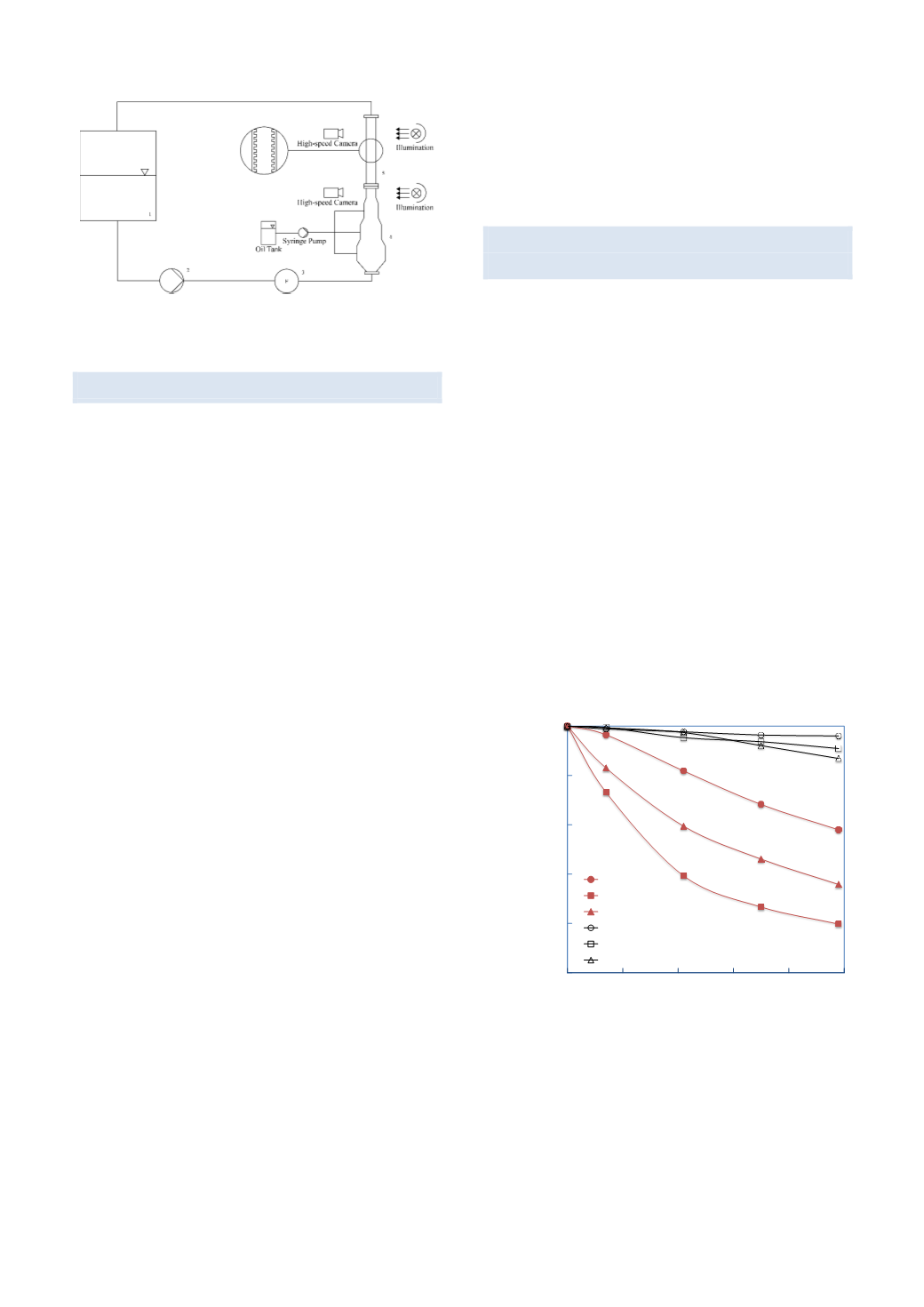
Fig. X Experimental facility for single particle breakage
investigations.
ACID GAS REMOVAL BY ABSORPTION
Removal of acid gases, like CO
2
and H
2
S using chemical
absorbents is a commercial technology used for decades.
However novel, environmentally friendly solvents with
low energy requirement during solvent regeneration are
needed. Our work is concentrated along two axes, one
studying CO
2
capture from off gases from fossil fuel
power plants as well as from the iron and steel-making
industry, and the other directed toward the removal of
acid gases from natural gas.
We have experience with theoretical screening by use of
computational chemistry, experimental screening and
solvent development, characterization of equilibria,
thermal properties, transport properties and kinetics
and testing in a laboratory pilot plant. In parallel we
develop rigorous thermodynamic models and improved
models for combined mass and heat transfer. In addition,
we have developed together with CO
2
Capture Process
Technology group at SINTEF MK a full rate based
simulator for the whole absorption/desorption process,
CO2SIM. Surplus to these activities, we have been
working on solvent degradation and corrosion as well as
environmental issues.
We have many research projects in this area funded by
the Research Council of Norway, the industry, and the
European Union. Our work is concentrated along two
axes, one studying CO
2
capture from off gases from fossil
fueled power plants and industry, and the other directed
toward the removal of acid gases from natural gas. We
were heavily involved in EU FP6 projects, e.g CASTOR and
CAPRICE. This work continued in the EU FP7 CESAR and
as coordinator of the EU FP7 iCap project. Currently we
are partner in the OCTAVIUS and HiPerCap projects. Of
the current national projects on CO
2
capture, the largest
is
SOLVit
, a JIP with Aker Solutions and SUBPRO.
Furthermore, we leading national project
3
rd
Generation
membrane contactors
(3GMC), and are partner in
following two national projects:
Low energy penalty
solvents
(LEPS) and
Magnetic separation of CO
2
through
sorption on magnetic hybrid nanoparticles
(CARBOMAG).
COMBINED SUBSEA HYDRATE CONTROL AND H
2
S
REMOVAL (SUBPRO)
Pipelines used to transport produced gas have quality
restrictions related to content of water, CO
2
, H
2
S and
heavy hydrocarbons. If these requirements cannot be
met, oil wells may need to be closed. Today on a typical
platform water is removed by Triethylene glycol and
amine processes are used to remove CO
2
and H
2
S. In
addition to this Monoethylene glycol is used for hydrate
control in the well flowlines, giving in total 3 different
chemical systems with separate absorption and
regeneration equipment. Simplifying the chemical
systems or moving equipment and process elements
subsea could be a way to ensure better energy efficiency
and utilization of the resources.
We are working on developing a new regenerative
process where both hydrate formation is controlled and
H
2
S removed. Since this would be a regenerative process
significantly higher concentrations of H
2
S could be
treated than normally is the case with scavengers.
Amine concentration as a function of time during thermal
degradation experiments.
0%
20%
40%
60%
80%
100%
0
10
20
30
40
50
Amine conc (remaining)
Days
MEA+H2O
MEA+MEG
MEA+TEG
MDEA+H2O
MDEA+MEG
MDEA+TEG


















