
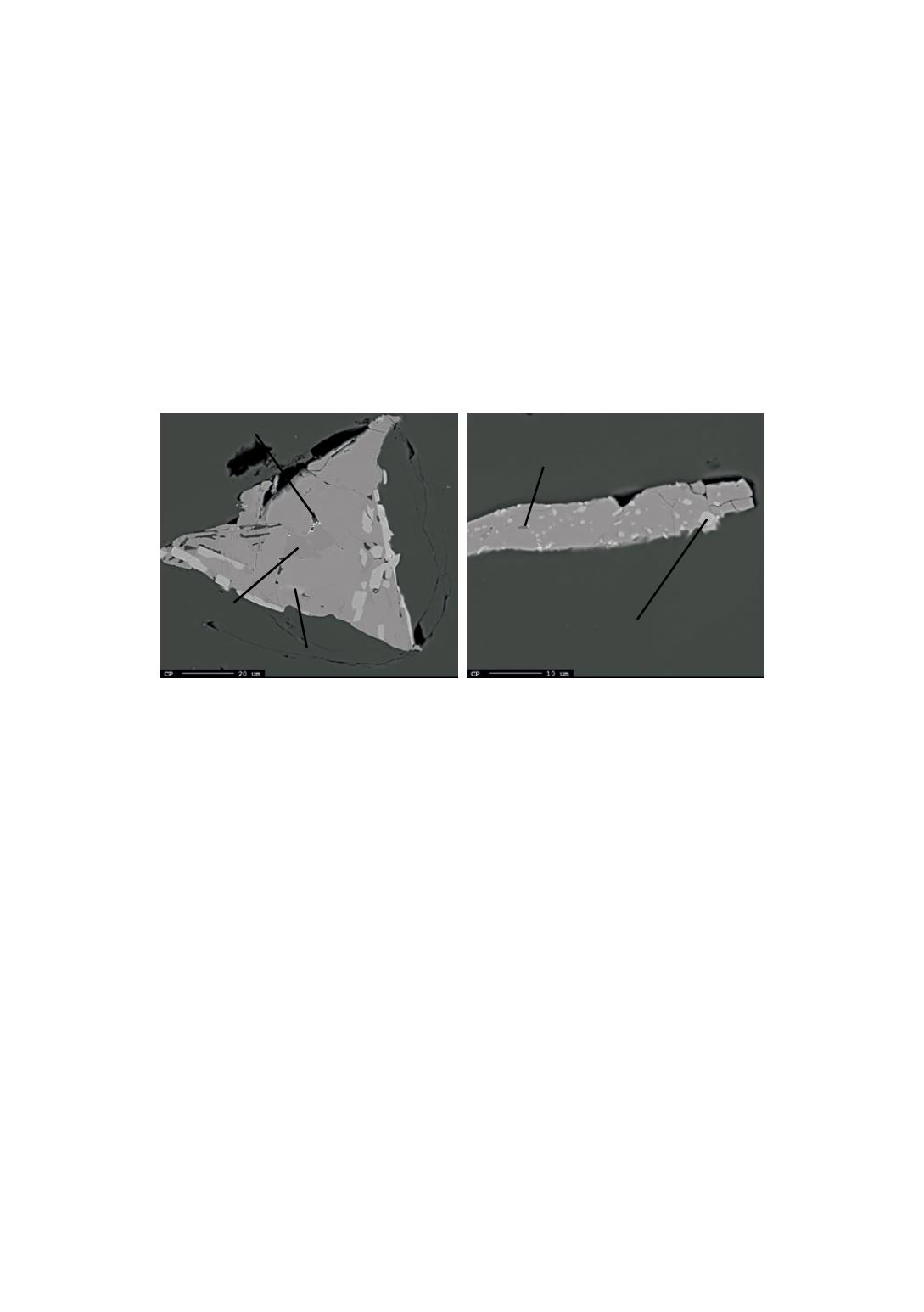
3.2.2 Sample annealed at 1200°C
Figure 5 shows two intermetallics found in the annealed sample. FeSi
2
is still the main
compound present in the particles. HT-FeSi
2
was not found. Si precipitates and cracks
are more frequent in this sample rather than in the other two. Their quantity increases
where LT-FeSi
2
is detected. Al
6
CaFe
4
Si
8
was reduced in quantity, as predicted by
SiStruc
®
. This causes TiFeSi
2
to be the second most common intermetallic in the
annealed sample. CaAl
2
Si
2
was not found in any particle chosen, even if it was
expected by SiStruc
®
to be the second most present phase. Al
8
Fe
5
Si
7
occupies larger
areas compared to what was seen in the untreated material. TiFeSi
2
changes its
features about the particle size distribution. It is more common to find high number of
TiFeSi
2
particles, with irregular shapes and small areas. Some particles kept the
rectangular microstructure anyway.
Figure 5
: BSE images coming from the center (a) and bottom (b) of the sample annealed at
1200°C.
4. Discussion
4.1 Non-equilibrium effects: untreated sample
SiStruc
®
is a software merely based on thermodynamics. Solidification is far from
equilibrium in this case. Data about %wt of different elements have to be handled
carefully. Non-equilibrium conditions might have caused deviations from the
previsions calculated by SiStruc
®
. TiSi
2
and Al
3
FeSi
2
were expected to be found at
least in low quantities in the untreated sample, whereas none of them was found.
Besides, Al
6
CaFe
4
Si
8
is found in higher quantity than CaAl
2
Si
2
.
Having a look at the cooling ranges can explain these differences. Figure 6 shows
the cooling ranges calculated by SiStruc
®
for the composition of the untreated sample.
FeSi
2
will be the first compound which solidifies. It will be the most present because
of the high content of Fe in the material. TiSi
2
and TiFeSi
2
will form at the same
point, but previous works have verified that TiSi
2
-formation reaction is hindered when
there is a high presence of Fe [3,17]. CaAl
2
Si
2
forms at a lower temperature compared
to Al
6
CaFe
4
Si
8
. Ca will be involved in the quaternary phase transformation first. What
is left will start reacting with Al and Si to form CaAl
2
Si
2
. It can be hypothesized that
the formation of Al
6
CaFe
4
Si
8
has a faster kinetics than the formation of CaAl
2
Si
2
.
FeSi
2
Al
8
Fe
5
Si
7
Si precipitate
TiFeSi
2
Al
6
CaFe
4
Si
8
FeSi
2
Si precipitate
89


















