
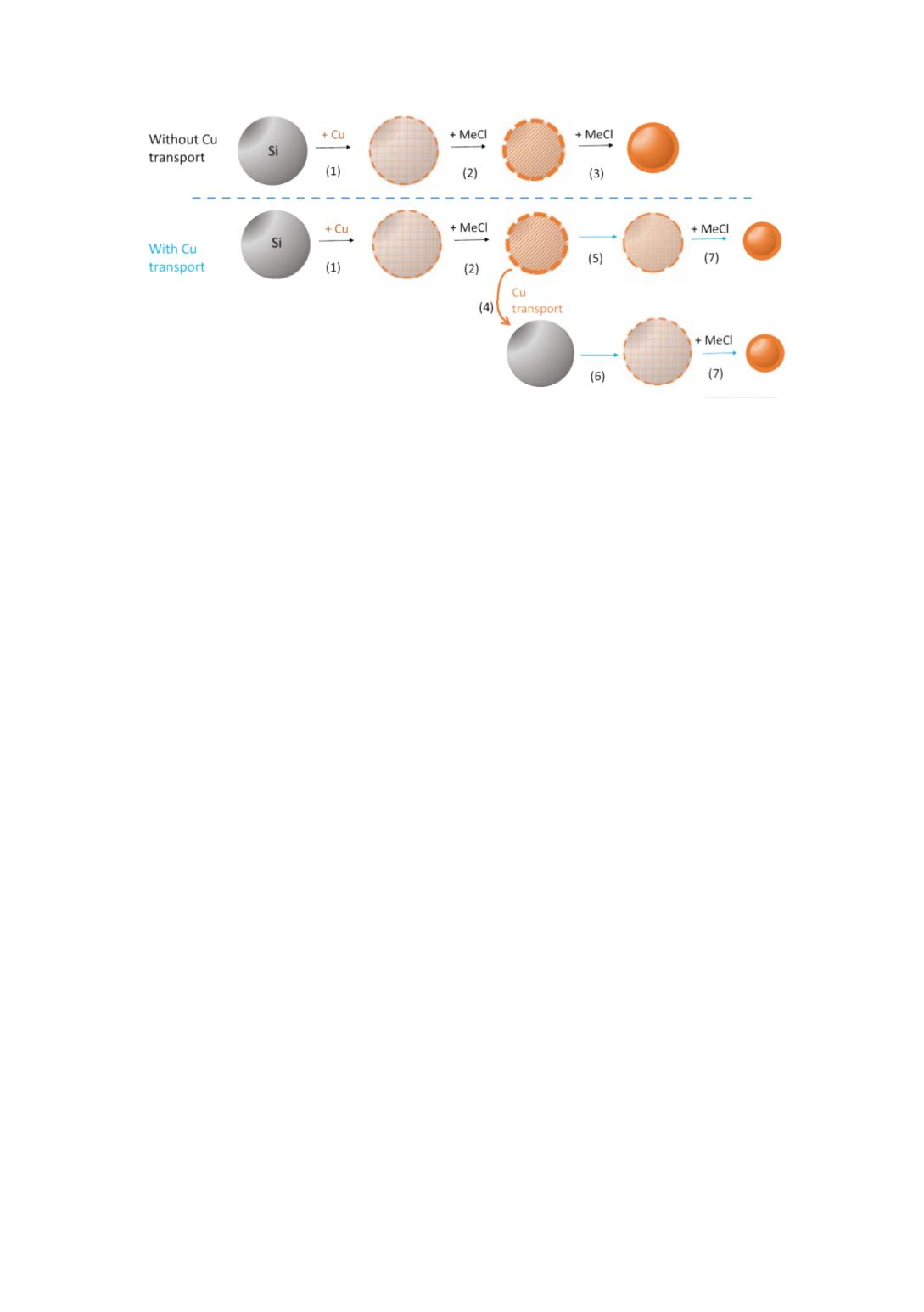
Figure 3
: Silicon particle evolution with or without copper transport
After initiation (1), activated silicon particles is consumed by reaction with methyl
chloride (2) until the catalyst layer at the silicon surface is too thick and prevent
methyl chloride and silicon contact. If copper is transported from an activated to a
fresh silicon particle (4) catalyst concentration is decrease at the initial particle surface
(5) enable further conversion of the silicon particle (7). In the same time a new silicon
particle is activated (6). The mechanism can be continuously repeated.
Very few documents describe the transport phenomenon in the MCS reactor.
Some work concerning catalysts movement in the FBR was done to understand poor
performances obtained at industrial scale with low aluminium silicon [13-14].
Hypothesis to explain the bad performances was the lack of catalyst movement in the
FBR, in relation with low Al content. Experiments performed at lab scale consisted in
vaporizing CuCl, ZnCl
2
, and mix of them together with AlCl
3
or not. AlCl
3
was
proved to favour vaporization of copper and zinc chlorides, by forming ZnCl
2
-AlCl
3
or CuCl-AlCl
3
adducts with low boiling temperature.
In order to maintain stable performances in the industrial FBR and avoid particle
deactivation, it seems necessary to have homogeneous and low copper concentration
at the silicon particles surface. Good catalyst transport from activated to fresh silicon
particles should contribute to establish these conditions. The objectives of the study is
to better understand parameters influencing the catalyst transport from activated to
non activated silicon particles and the performances of the catalytic sites resulting
from this transport. Specific experiments were designed for this purpose.
128


















