
(Me
2
SiCl
2
) is the main product of the reaction but it is produced together with many
by-products.
...
,
,
,
,
2
2
3
3
2
2
, ,
)(
HSiCl
Me
MeHSiCl
SiCl
Me
MeSiCl
SiCl
Me
MCS
g
highboilin
lowboiling
MCS
MeCl
Si
g
g
g
Sn Zn Cu
g
s
Industrially, this heterogeneous and highly exothermic reaction (
Hr = -284.1 KJ/mol
if we take only Me
2
SiCl
2
into account), takes place in a fluidized bed reactor (FBR)
under a pressure of between 2.5 and 4 bars and a temperature around 300°C. Common
objective of the MCS producers is to perform the reaction with a high level of
productivity, a high selectivity of Me
2
SiCl
2
and good silicon utilization.
Despite numerous studies and publications, today the mechanism of the direct
synthesis of MCS has not been fully understood. Particularly for this reaction, is that
the catalyst and promoters are supported on silicon, which is a reactant [4].
The performances of the reaction will depend on the initiation step (active sites
formation though reaction between silicon and copper), on the evolution of the
contact mass (silicon surface is constantly changing due to silicon consumption by the
reaction) and on the ability of the system to regenerate. Copper transport from
activated particles to fresh silicon is key to maintain stable performances.
Bibliographic review
In a batch mode, performances of the direct synthesis will depend on the initiation
step. This step has been extensively studied at lab scale since the fifties [5-8]. In this
step, chemistry and hydrodynamics are strongly interlinked. Main drivers for the
initial performances are silicon chemical quality and particle size distribution
(PSD),
catalysts and cocatalysts chemical quality and PSD, pressure, temperature, quality of
the mixing. At the end of the initiation, stable performances in term of productivity
and selectivity are obtained in the reactor.
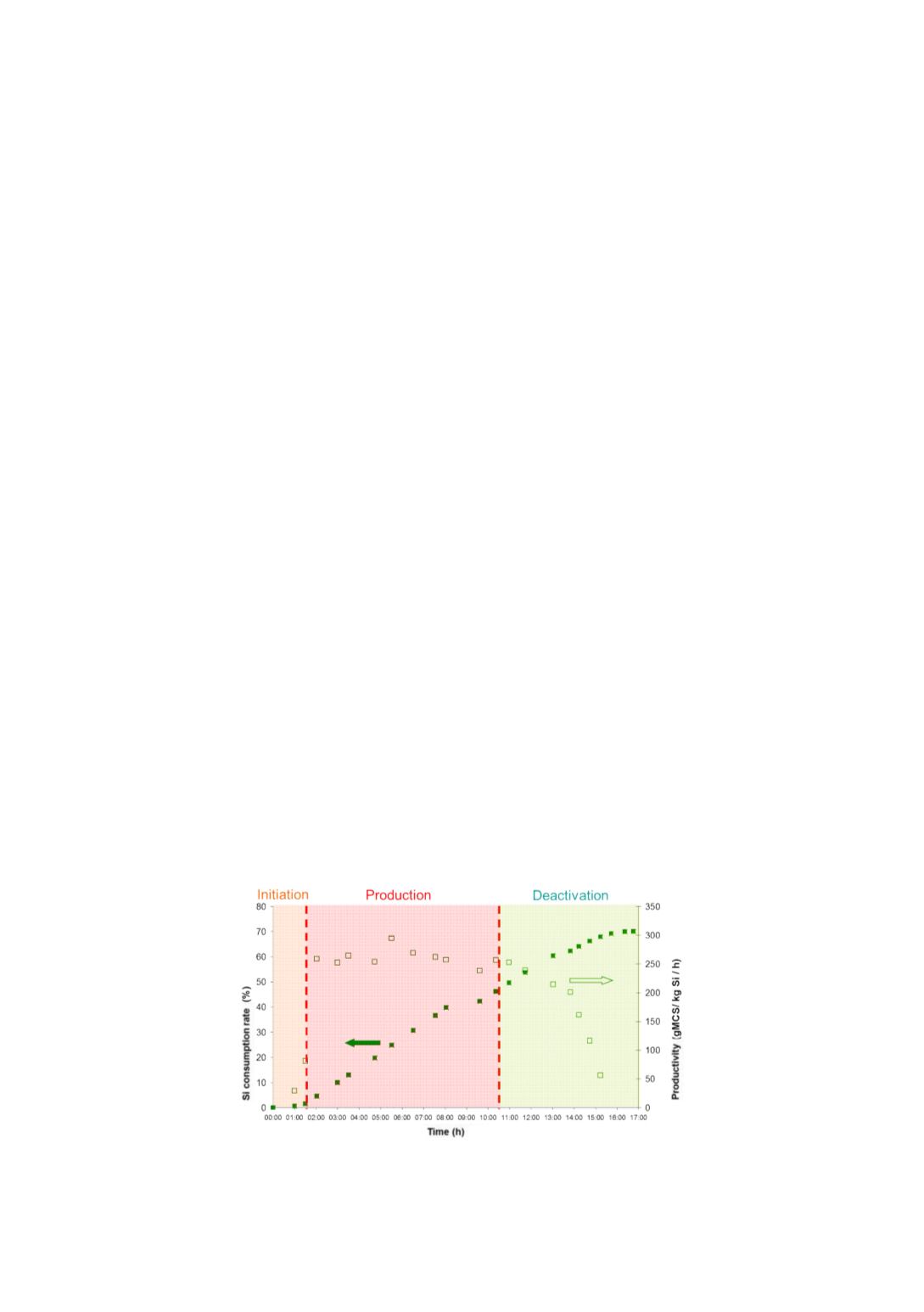
In batch mode, deactivation of the mass can be observed at lab scale: after
consumption of a part of the silicon, the performances start decreasing as represented
in the graph below.
Figure 1
: Silicon conversion and productivity evolution in MCS batch reaction
126


















