
temperatures. Cristobalite formation in one of the quartz sources, Qz 9 was therefore
investigated at higher temperatures by KjelstadliKjelstadli [12, 13]. The data from the
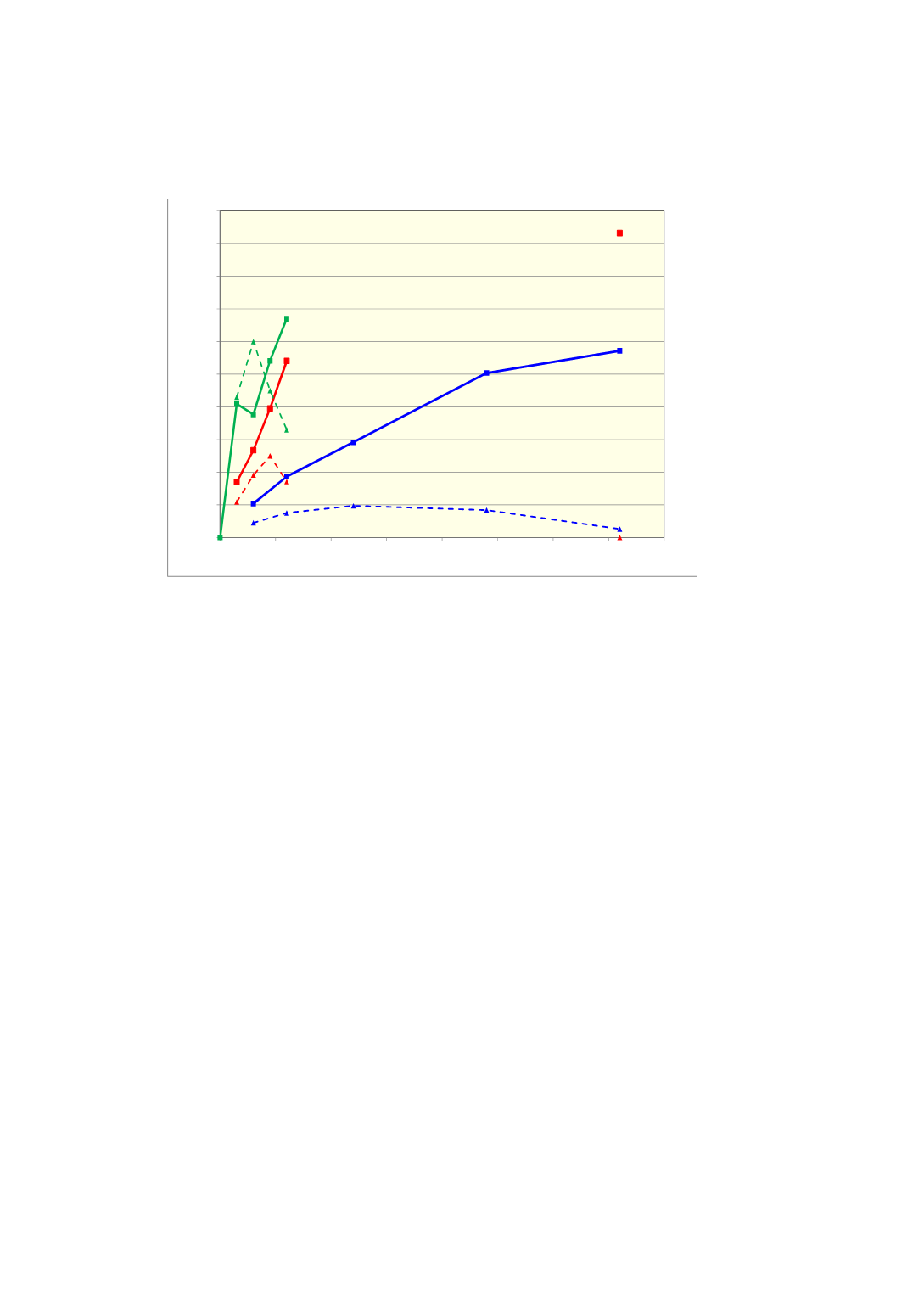
different investigations of Qz 9 are combined in Figure 5.
0
10
20
30
40
50
60
70
80
90
100
0
50
100
150
200
250
300
350
400
% of phases at 1500
o
C
Time, Minutes
1500
o
C
Cristobalite
1500
o
C
Amorphous
1600
o
C
Cristobalite
1600
o
C
Amorphous
1700
o
C
Cristobalite
1700
o
C
Amorphous
Figure 5
Effect of temperature and time for formation of cristobalite and amorphous
silica for one quartz source, Qz 9, heated in air. Investigations at 1500
o
C and at 1600
o
C after 6 hours by Ringdalen [8] and the remaining investigations from Kjelstadli
[12, 13]
When quartz Qz 9 is heated to 1600 °C and 1700 °C, amount of amorphous silica will
act similarly as when it is heated to 1500 °C, first increase and then decrease again,
while amount of cristobalite increase continuously. Transformation from
β
-quartz to
cristobalite thus seems to go through an amorphous phase at temperatures from 1500
0
C and higher. Maximum amount of amorphous silica and rate of formation of
cristobalite and amorphous silica increase with increasing temperature. Even at
temperatures as high 1700 °C, it takes more than 1 hour before the investigated
quartz, Qz 9 is completely converted to cristobalite. After 30 minutes, the sample
contains 60 % amorphous silica. It is hence likely that quartz at its melting
temperature of 1726 °C will contain some amorphous silica that may effect the
melting properties of the quartz.
The results in Figure 5 are based on quartz particles in the size 1-2.8 mm with an
average size of 1.9 mm. The effect of size was investigated by Kjelstadli [13] in the
same apparatus by using larger pieces, average sizes 33 mm and 42 mm. An
intermediate amorphous phase was as shown in Figure 6 also present during
cristobalite formation in these larger particles. In these investigations, only one
particle was used. The weight of one 33 mm particle is 50 gram, the same as the
weight used in the investigations of 1-2.8mm particles. One 42 mm particle weighs
100 gram, twice the mass used in the other investigations. Only one sample has been
tested for each of the conditions. Statistical data for the samples are thus not known
and the results are only indicative. Amorphous content in the 33 mm sample heated
for 20 minutes is contradictory to other results and in further discussions regarded as
an outlier.
274


















