
When heated in air at 1 atm
α
-quartz is transformed to
β
quartz at 573 °C. This
transformation is fast and will be reversed during cooling. It will lead to an increase in
volume of around 0.4% According to the phase diagram tridymite will be formed at
873°C. It is debated if this phase change really will take place or if
β
quartz
alternatively will be transformed directly to
β
cristobalite at a temperature somewhere
between the temperature for tridymite formation from the phase diagram and the
melting temperature for SiO
2
at 1711 °C. During this transformation, the volume will
increase with around 17%. When it is cooled,
β
cristobalite is transformed to
α
-
cristobalite and not back to the stable phase,
α
-quartz. The large increase in volume
by the phase transformations will give a lower density and a higher specific surface
area after heating. Cracking as a result of the volume change will contribute to the
higher surface area. The transformation from
α
-quartz to
β
-cristobalite is slow and in
experiment by Wiik [
7
], the quartz was heat treated at 1400
o
C for 5 days in order to
achieve complete transformation to cristobalite. The rate for the phase transformation
from
α
-quartz to
β
-cristobalite has as illustrated in
Figure 3
been shown to vary
considerably between different quartz sources [8,9]. With further heating, the quartz
will soften and melt. The melting temperature is 1726
o
C. During heating the quartz
will first start to soften, normally at a temperature lower than 1726
o
C, and not be
completely molten until the temperature is considerably higher than 1726
o
C. Melting
properties vary as shown in Figure 3 between quartz sources and depend on heating
rate. [8,10].
1500
1550
1600
1650
1700
1750
1800
1850
0
10
20
30
40
50
60
70
80
90
100
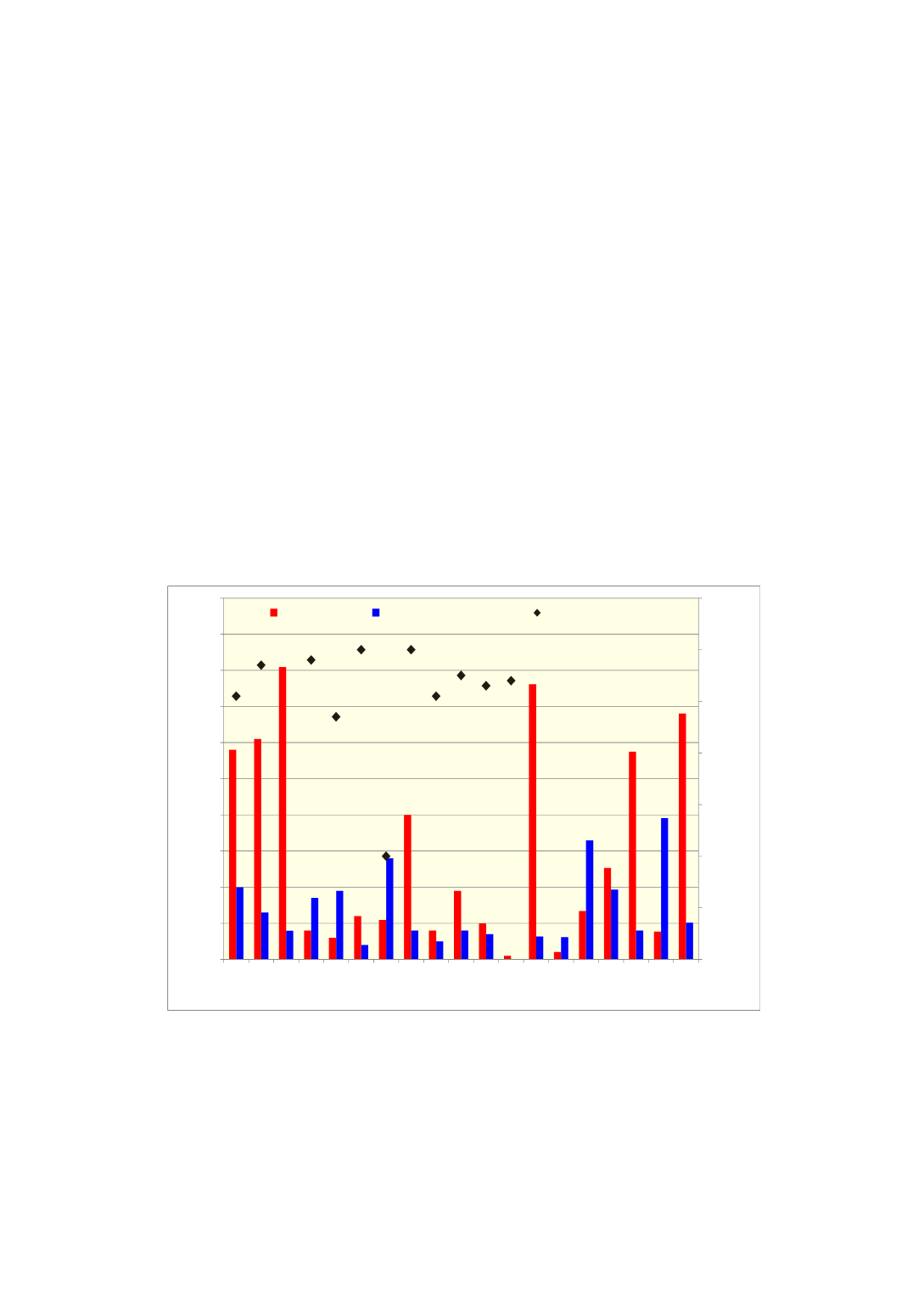
Qz1 Qz2 Qz2B Qz3 Qz4 Qz5 Qz6 Qz7 Qz8 Qz9 Qz10 Qz11 Qz12 Qz13 Qz14 Qz15 Qz16 Qz18 Qz17
Temperature
o
C
% of phases after 1 h heating at 1500
o
C
Quartz source (sample)
% Cristobalite % Amorphous silica in air
Softening temp in CO
Figure 3
Amount of cristobalite and amorphous silica in different quartz samples after
heating for 1 hour at 1500 °C. Softening temperatures for sample Qz 1-11 are also
included.[8]
Amount of cristobalite has earlier been proposed [8, 11] to be of importance for
reaction rate. Ongoing unpublished research has so far not given any conclusive
results regarding this. The
α
-quartz –
β
cristobalite transformation is as described by
Wiik [7] assumed to go through an amorphous phase. The amorphous phase is
272


















