
expected to be more reactive than the crystalline phases and might as indicated by
Doris [11] also affect the melting properties, possibly giving a lower softening
temperature. Amount of amorphous phases may thus be of importance for reactions
and performance in Si-furnaces. Appearance of the amorphous phase has in addition
to earlier investigations [8] recently been investigated as summer and student project
by Kjelstadli [12,13]. Results from these and earlier investigations are here
summarised and discussed.
Formation of cristobalite and amorphous phase were investigated by heating of quartz
in air in a rapid heating furnace by the method described by Ringdalen [8]. The
amounts of different phases in the samples were measured by quantitative XRD.
(Bruker D8 advance with the software Diffrac Plus Topaz). The amount of amorphous
silica was determined by the method described
in detail by Kjelstadli [13]. The main
principle is to add a known amount of a crystalline phase, and to calculate amount of
amorphous silica based on this known amount and the XRD measurements. The bias
in the measured results is calculated to ± 2 weight % (absolute %).
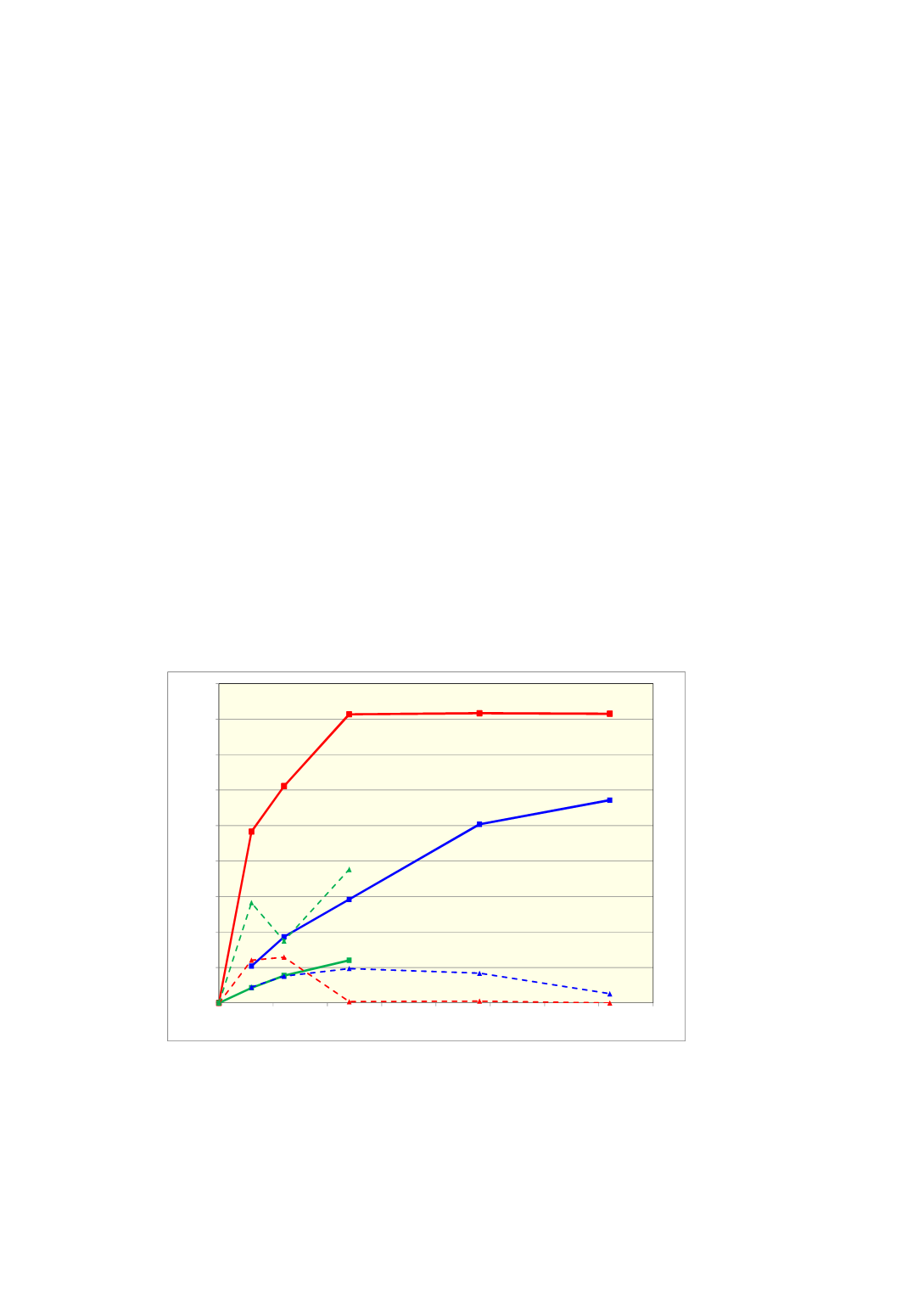
Amount of cristobalite and amorphous silica after a certain heating time were in
earlier investigations [8] found as illustrated in
Figure 3
to vary considerably between
different quartz sources. It was also as shown in Figure 4 found that amount of
amorphous phase first increased up to a level and the decreased again while amount of
cristobalite increased. The rate of formation of amorphous silica and its maximum
values also varied between different quartz sources. The described trend in amount of
amorphous phase is taken as a strong support to the theory [11] that cristobalite
formation goes through an amorphous phase.
Figure 4
Effect of time on formation of cristobalite and amorphous silica when
different quartz sources are heated in air at 1500 °C [8].
At 1500
o
C, transformation to cristobalite is relatively slow. In order to evaluate its
effect of reactions with silica, it will be important to know if the intermediate
amorphous phase is formed also when the transformation takes place at higher
0
10
20
30
40
50
60
70
80
90
0
50
100
150
200
250
300
350
400
% of phases at 1500
o
C
Time, Minutes
Qz2
- Cristobalite
Qz9
- Cristobalite
Qz3
- Cristobalite
Qz2
- Amorphous
Qz9
- Amorphous
Qz3
- Amorphous
273


















