
Highest levels of reactivity were obtained mainly in the experiment at room
temperature without any addition and in the one with Al addition. In both cases,
copper and promoters were at low level in the fraction from day 2.
As reactivity seems to be more correlated with the level of cocatalyst we tried to
establish simple correlations between cocatalyst amount in the fraction + 100µm and
the silicon consumption rate of this fraction.
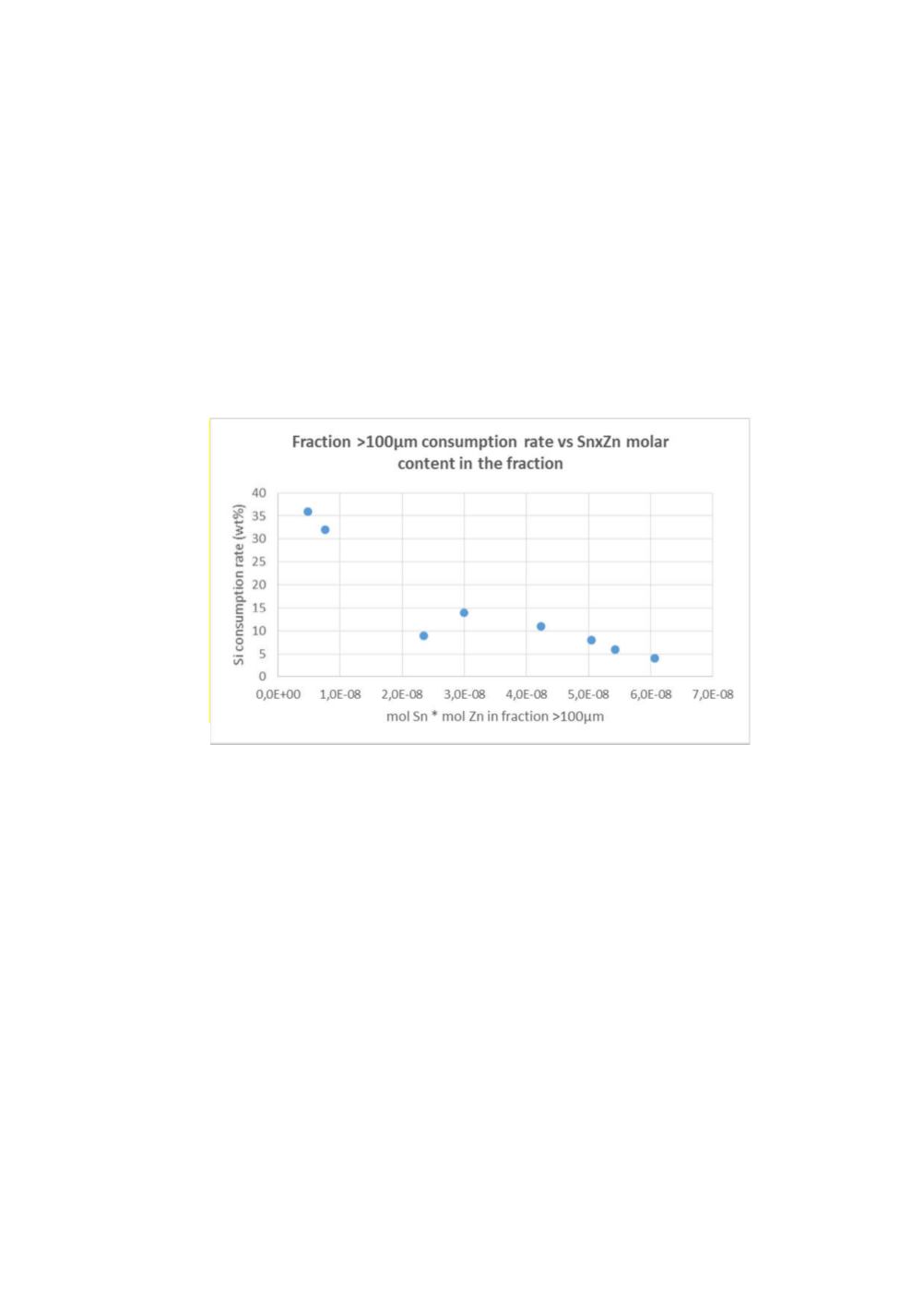
Inverse correlation could be found between silicon consumption rate of the fraction
+100µm and the product of tin and zinc molar content in this fraction, indicating that
high concentrations of cocatalysts seems detrimental for the reactivity of the fraction
+100µm.
Figure 7:
Fraction +100µm consumption rate versus (Sn*Zn) molar content in this fraction
If tin and zinc are necessary to initiate the reaction between silicon and catalyst, their
role in catalyst transfer is less clear. It seems that the transfer of copper is not
correlated to the transport of cocatalyst; Cocatalyst do not seem to contribute to
copper transport.
Cocatalysts in the fraction + 100µm seem to act as a poison for the direct synthesis
reaction. Surface concentration of tin and zinc in this fraction are much higher than
standard conditions and the ratio Sn / Cu and Zn / Cu are much higher as well.
Poisonous effect of cocatalysts used at too high concentration is described in the
literature [15].
137


















