Basic HTML Version
120
For a monovalent salt such as NaCl or KCl the ionic strength equals the molar
salt concentration. The situation changes for salts having more than one
charge (z>1). The example here is 0.05 M Na
2
SO
4
:
I
0.05M Na
2
SO
4
=
1
2 2
⋅
0.05
⋅
1
2
+
0.05
⋅
2
2
(
)
=
0.15
The divalent sulphate ions contribute more than monovalent ions due to the z
2
factor.
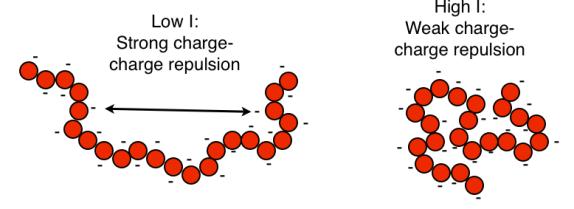
The polyelectrolyte character and the role of the ionic strength can easily be
observed by monitoring the extension of the chains at different ionic strengths:
The negative charges along the chain results in intramolecular electrostatic
repulsion, thereby expanding the chain (increasing electrostatic contribution to
the expansion coefficient
α
2
).
The repulsive force between two equal charges (and hence
α
2
) depends on:
a) The distance between the charges
b) The ionic strength
The Debye length (
κ
-1
) is a quantitative measure of how far the electrostatic
influence can be detected. Wikipedia uses the definition ‘
the measure of a
charge carrier's net electrostatic effect in solution, and how far those
electrostatic effects persist
’. The Debye length is a function of the ionic
strength:
κ
−
1
=
8
π
e
100
ε
kT
⎛
⎝⎜
⎞
⎠⎟
−
1
2
I
−
1
2
Symbols: e is the electronic charge,
ε
is the dielectric constant of the solvent.
For water at 25
°
C a 1:1 electrolyte has a Debye length given by:

