Basic HTML Version
119
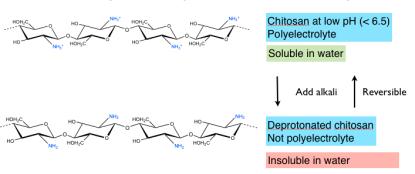
contrast to alginates, which precipitate by acidification, chitosan precipitates
by increasing pH above ca. 5.5 (pK
A
of the amino group) and dissolves again
upon acidification.
These examples show that polyelectrolyte properties are intimately related to
acid-base properties, as charges can be turned on and off simply by varying
pH (acid-base titrations). We therefore need to review acid-base
fundamentals (below).
The polyelectrolyte character is also easily observed in solution, as the
viscosity of the biopolymer solution becomes highly dependent on the ionic
strength, as explained in subsequent sections.
3.1.5. Polyelectrolyte effects: Role of
ionic
strength.
The ionic strength is a fundamental parameter in polyelectrolyte theory. It
enters many equations, accounting for the influence of added salts, including
the strong effects of higher valencies. In general, two different salts such as
NaCl and Na
2
SO
4
have the same physical effects as long as they have the
same ionic strength, not the same molar concentration. To obtain an ionic
strength of 0.1 one needs either 0.1 M NaCl or 0.033 M Na
2
SO
4
. This follows
from the definition of the ionic strength (I):
I
=
1
2
C
i
z
i
2
i
∑
Here, C
i
is the molar concentration of each ionic species, and
z
is its valency
(number of charges). Using 0.1 M NaCl as an example we obtain:
I
0.1M NaCl
=
1
2
C
i
z
i
2
i
∑
=
1
2 0.1
⋅
1
2
+
0.1
⋅
1
2
(
)
=
0.1

