Basic HTML Version
121
κ
−
1
water, 25
°
C
=
0.304
I
−
1
2
⎛
⎝⎜
⎞
⎠⎟
(nm)
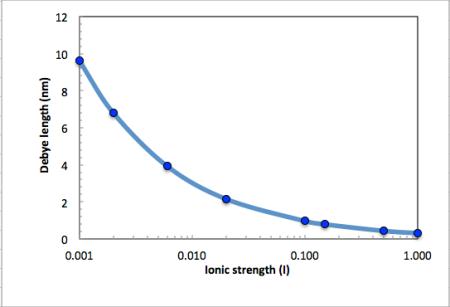
The Debye length (in water) and hence influence of charges decrease rapidly
with increasing ionic strength as shown in the figure below.
A quantitative parameter reflecting the chain expansion and which can be
easily and directly observed is the radius of gyration (R
G
), which can be
obtained by light scattering. Another possibility is the solution viscosity, which
is intimately related to R
G
(explained more detailed in Section 6.1). Hence, the
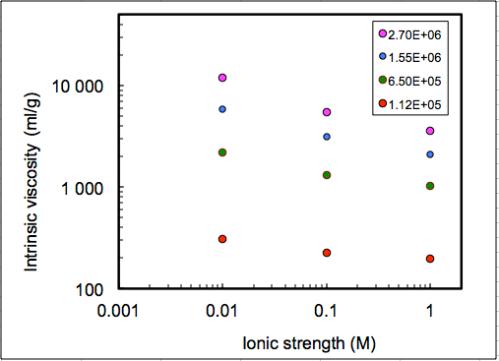
solution viscosity of water-soluble polyelectrolytes is generally very dependent
on the ionic strength.
A practical consequence is, for example, that a polymer gives much lower
solution viscosity when dissolved in seawater than in pure water. The extent
to which the chain extension responds to changes in ionic strength is
determined by two factors:
The inherent stiffness of the chain (Effect of C
∞
): Very stiff chains do not
respond to changes in ionic strength, whereas flexible polymers may expand
or contract depending on the intramolecular attractive or repulsive forces.
The electrostatic repulsion between equal charges along the chains (Effect of
α
2
): High charge density (number of charges per sugar residue) increases the
repulsion, whereas high ionic strength reduces charge-charge interactions.

