Basic HTML Version

40
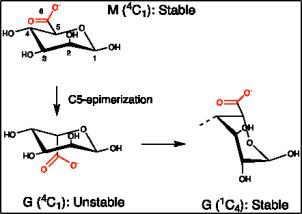
We will investigate the chemical and physical basis of such changes. Like
almost all commonly occurring carbohydrates, for example D-glucose,
β
-D-
mannuronic acid residues exist in the
4
C
1
ring conformation. This means that
the –OH groups at C1 (linked to next sugar), C3, C4 (also linked) and the –
COO
-
group are equatorial, whereas the –OH group at C2 is axial. Axial
substituents are energetically unfavourable, but in this case a singe axial –OH
is not enough to change the ring conformation. However, when the C5-
epimerases – when converting M to G - change the configuration at C5, the
bulky carboxylate group becomes axial. This results in an unstable (high
energy) situation, and in return the sugar ring flips to the alternative
1
C
4
conformation. Such ring flipping generally swaps the axial/equatorial status of
the substituents. In consequence, hydroxyls at C1, C3 and C4 now become
axial, whereas the –COO
-
(C6) and the –OH at C2 become equatorial.
The
4
C
1
to
1
C
4
transition has immediate consequences for the linkage
geometry:
•
MM: diequatorial (
eq-eq
)
•
MG: equatorial-axial (
eq-ax
)
•
GM: axial-equatorial (
ax-eq
)
•
GG: diaxial (
ax-ax
)
This is further illustrated in the figure below (..GGMM..):
Figure 18. The
4
C
1
to
1
C
4
transition following epimerization in alginates

