Basic HTML Version

33
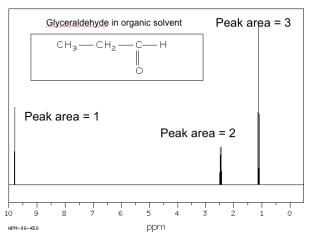
The spectrum of glyceraldehyde consists of three signals only, where the
peak area is 1:1:3, reflecting the 3 types of protons in the molecule.
In addition to chemical shifts NMR provide a wealth of information:
Spin-spin coupling leads to splitting of peaks. They appear typically as
singlets, doublets, triplets or quartuples depending on the number of H-atoms
on adjacent carbons. The coupling constants provide detailed chemistry and
torsion angles.
Spin relaxation: The decay of magnetization depends on the molecular
dynamics. It can be used to find the diffusion constant of e.g. a protein in
solution.
More advanced NMR methods exist, enabling for example to determine the 3-
D structure of proteins.
The use of
1
H-NMR in the structure determination of carbohydrates in general
and alginates in particular will be illustrated by the following examples.
1.2.9. The
1
H-‐NMR
spectrum of D-‐glucose
(in D
2
O):
The purpose of using D
2
O (heavy water) is to exchange –OH protons with –
OD:
R-OH + D
2
O (excess) = R-OD + HDO
HDO protons give a large peak at 4.8 ppm, but deuterated hydroxyls are
‘invisible’ by NMR and simplify the NMR spectrum significantly. Still, the
spectrum is complicated because we observe all C-linked protons in glucose
(one proton each at C1, C2, C3, C4 and two protons at C6):
Figure 10.
1
H-NMR spectrum of glyceraldehyde

