Basic HTML Version
131
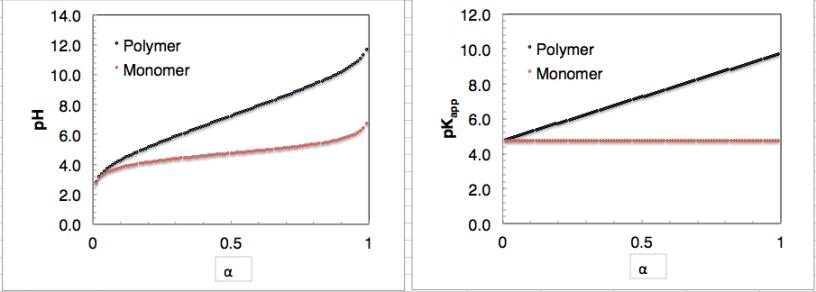
Titration of hyaluronan (figures further up) at different ionic strengths further
shows:
a)
pK
a
decreases with added salt
b)
pK
a
becomes less dependent on
α
with added salt.
In the limit
α→
0 and I
→∞
hyaluronan has an intrinsic pK
a
of 2.9
36
The influence of the ionic strength on pK
a
is straightforward and logic: Higher
ionic strength reduces the influence of the charges, which disappear
completely at infinite ionic strength.
3.1.10. Titration of
chitosan: A polycationic polysaccharide
The acidic form of chitosan is at the same time the ionized form:
In this case the degree of ionization becomes equal to 1-
α
since the
dissociation of positively charged –NH
3
+
results in a neutral –NH
2
group.
Titration with NaOH gradually reduces the number of charges until the
chitosan becomes neutral, which also leads to precipitation (with a few
exceptions).
The following example shows the titration of 4 chitosans using the
electrophoretic mobility as a quantitative measure of the charge:
36
Cleland et al. (1982) Macromolecules 15, 386-395
NH
3
+
O
HOH
2
C
NH
3
+
HO
O
O
HOH
2
C
HO
O
NH
3
+
O
HOH
2
C
NH
3
+
HO
O
O
HOH
2
C
HO

