Basic HTML Version
128
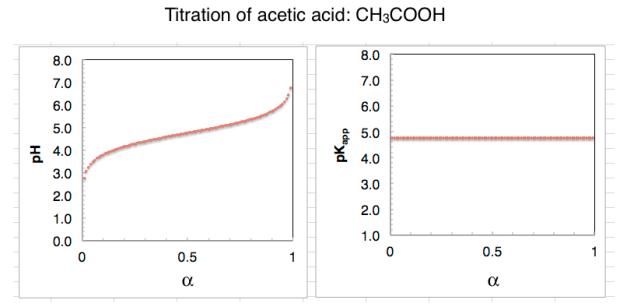
Consider then a dibasic acid such as oxalic acid:
Oxalic acid contains two chemically identical carboxyl groups. Yet, oxalic acid
has two pK
a
values: 1.25 and 4.14 (Wikipedia). This means the first ionisation
(H
2
A
→
HA
-
) proceeds easily (low pK
a
), whereas ionisation the second
carboxyl is less favoured (high pK
a
). This can be understood by the influence
of the charges. Forming a –COO
-
in close proximity of an existing negative
charge is thermodynamically unfavourable. The titration curve of oxalic acid
clearly reveals the two pK
a
values.
HO
OH
O
O
HO
O
O
O
O
O
O
O
H
2
A
HA
-
A
2-

