Basic HTML Version
117
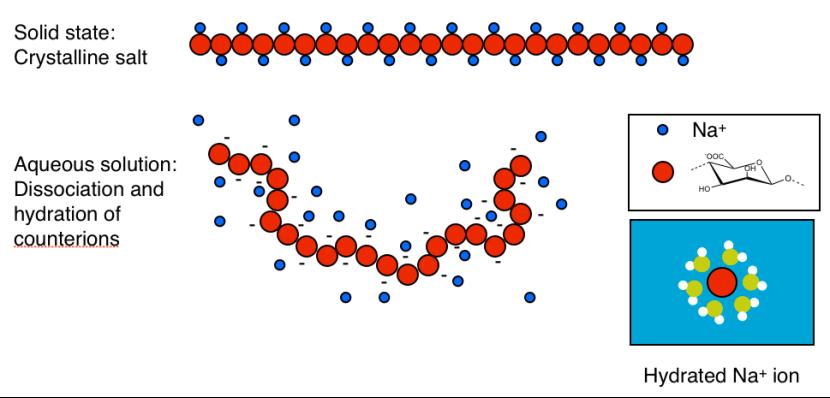
Sodium alginate is a typical example. In the dry state each carboxylate group
(-COO
-
) is bound to a sodium ion (Na
+
). In other words, sodium alginate is the
sodium salt of alginic acid. Upon dissolution in water the sodium ions
dissociate from the polymer to become hydrated, free ions, but remain
concentrated around the polymer as illustrated below.
The counterions may alternatively be any other cation such as K
+
, Mg
++
, NH
4
+
or Ca
++
. The proton (H
+
) is the counterion for alginic acid, but since most of
the carboxylic acids are weak acids (pK
a
= ca 3.5 for alginic acid) only a small
fraction of the protons actually dissociates.
3.1.3. Changing
counterions
(salt
forms)
Interconversion between different salt forms is an important process
frequently applied to obtain other salt forms. Alginates are manufactured not
only as sodium alginate, but also as alginic acid, calcium alginate and
ammonium acetate (see e.g. E400-E405). The conversion may be obtained
by dialysis, or via the acidic form. The latter is more easily scaled up.
Example (sodium alginate to potassium alginate):
1.
Na-alginate is dissolved in water
2.
An acid (HCl or H
2
SO
4
) is added until pH reaches 1-2 (all –COO
-
protonated), forming insoluble alginic acid which is then washed with
water to remove excess ions
3.
pH is adjusted back to ca 7 using KOH, forming soluble K-alginate,
which can be dried if necessary.

