Basic HTML Version
10
1.1. CARBOHYDRATE FUNDAMENTALS:
MONOSACCHARIDES
Below is a brief overview of the basic rules in carbohydrate nomenclature, and
how to interconvert between Fisher and Haworth formulae. The topic is also
discussed in greater detail in chapter 4 of the textbook
1.1.1. The
Fisher projection
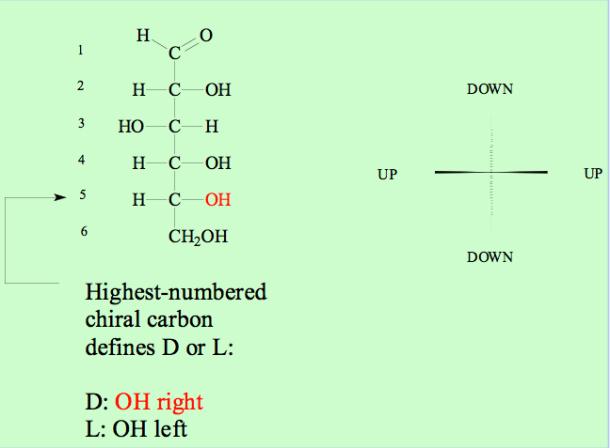
The Fisher projection is standard tool in organic chemistry to specify the
stereochemistry at asymmetric carbons, which are abundant in
carbohydrates.
Note the rule for distinguishing D- and L-sugars: The stereochemistry of the
highest-numbered chiral carbon defines D or L.
For hexoses (six carbon sugars) this corresponds to C-6, and for pentoses
(five carbon sugars) C-5.
1.1.2. D-‐ and
L-‐sugars
D- and L-sugars having the same name (e.g. D- and L-glucose) are
enantiomers. They are mirror images and cannot superimpose after rotation
or translation.

