Basic HTML Version
98
2.2.5. The R
G
-‐M
relationships
for
randomly
coiled
chains.
Biopolymers behaving like flexible chains in solution are common:
•
Most denatured proteins (-S-S- bridges cleaved, high T
31
, 6 M urea..)
(See also Section 2.2.13)
•
Denatured (single-stranded) DNA, RNA (high T)
•
Many linear (unbranched) polysaccharides:
•
Pullulan (very flexible)
•
Alginates (Na
+
-form, no Ca
+
)
•
Chitosans (pH < ca. 6)
•
Hyaluronan
•
Amylose (in DMSO or alkali)
•
Cellulose (in special cellulose solvents)
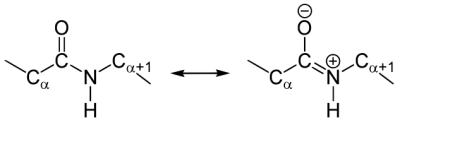
The flexibility is caused by the properties of the linkages between monomers.
In proteins, the peptide linkage has a certain freedom of rotation (Figure 9).
This applies in particular to the C
α
-C and N-C
α
linkages, whereas the partial
C=N double bond is much more rigid due to resonance:
Figure 41
In polysaccharides, the sugar rings are usually very rigid, but some rotation is
allowed for the glycosidic linkages (figure 10):
Figure 42
In total, even a small amount of rotational freedom in each linkage gives rise
to flexible chains.
It is important to realize that flexible chains do not adopt a single, fixed shape
or conformation. Due to thermal forces, the chains will constantly change
31
There are exceptions (cold denaturation)
OH
O
-
OOC
OH
HO
O
O
-
OOC
HO
O

