Basic HTML Version
84
It is convenient and common to use the weight fraction (w
i
) to quantify the
relative amounts of each chain length:
w
i
=
m
i
m
i
i
∑
Here, m
i
is the mass of each chain (i = DP).
Discrete distributions such as that in the figure above may be analyzed by
various mass spectrometry methods, especially for distributions that are not to
broad and DP is not too high (say, less than 100).
An equivalent way of describing the chain length distribution is in terms of the
number of molecules, i.e. the mole fraction. Converting weights (w
i
) to moles
(N
i
) and mole fractions (n
i
) is trivial:
w
i
=
N
i
M
i
n
i
=
N
i
N
i
i
∑
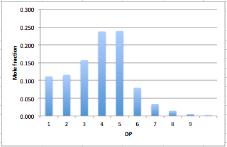
The sample above has the following distribution in terms of mole fractions:
Note how small molecules dominate in terms of numbers (moles), whereas
large molecules dominate the weight distribution (grams)

