Basic HTML Version

52
1.3.7.
Interactions with polyanions
(polyelectrolyte
complexes)
Polycations such as chitosans interact with polyanions to form polyelectrolyte
complexes (PECs). Examples include alginate-chitosan or DNA-chitosan. The
latter can be tailored to form nanoparticles, which are intensively studied as
non-viral gene delivery vehicles.
The basis for such interactions is the general interaction between oppositely
charged polymers. This depends on pH as illustrated for alginate-chitosan:
Alginates: pK
A
= ca. 3.5, below which alginates are neutral and do not
interact. Above this value alginates are polyanionic (negatively charged)
Chitosans: pK
A
= ca. 6.5, above which chitosans are neutral – and do not
interact. Below this value chitosans are positively charged.
It follows that alginate-chitosans PECs can only form between pH 3.5 and 6.5.
Which pH-range would allow DNA-chitosan PECs?
1.3.8.
Solubility
of
chitosans
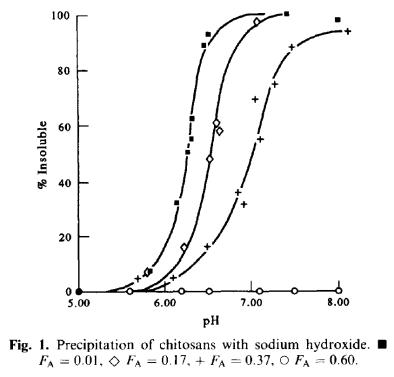
The solubility in water of high molecular weight chitosans is primarily
governed by the polyelectrolyte properties, in other words, the number of
charges. At high pH, where there are few or no positive charges, chitosans
are insoluble in water. However, as pH is lowered below pK
A
the charge
density increases towards it maximum (at pH = pK
a
– 1 chitosan has 90% of
Figure 32. The solubility of chitosans depends primarily on pH, but also F
A
and
molecular weight. Reproduced from Vårum et al. (1994). Carbohydr. Polym. 25,
65-70

