Basic HTML Version

42
1.2.15. Alginate, alginic acid: different
salt
forms
We can easily manipulate the salt form of alginate, meaning (ex)changing the
counterion, which accompanies the negatively charged carboxylate group to
ensure macroscopic electroneutrality. Most commercial alginates are Na-
alginate.
The carboxylate group has a pK
a
of about 3.5. By lowering pH below pK
a
we
obtain alginic acid:
-COO
-
+ H
+
= -COOH
In alginic acid essentially all carboxylate groups are protonated. To obtain
99% -COOH we must go 2 pH units below pK
a
13
Alginic acid is insoluble in water, and acidification is a very convenient way to
isolate alginate from an aqueous solution.
Neutralization of alginic acid by the appropriate base is a convenient method
to obtain any type of alginate (Na
+
, K
+
, (NH
4
)
+
, Li
+
, Mg
++
etc):
-COOH + KOH = -COO
-
K
+
(potassium alginate)
and similarly for other salts.
Other methods such as dialysis or cation exchange chromatography are also
used.
1.2.16. Size and
shape of alginate molecules
in
solution
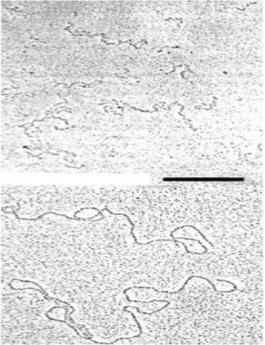
The overall shape and extension of alginate molecules are illustrated in the
following electron micrograph, depicting alginate
chains adsorbed to a mica surface. The scale bar
represents 200 nm, and since one sugar residue is
about 0.5 nm, 200 nm corresponds to about 400
sugars. Fully stretched, some of these alginate
molecules approach a contour length of 1000 nm =
1
µ
m or longer. This corresponds to 2000
monomers and a molecular weight of 2000 x 198 =
396.000 (Da = g/mol). Many industrial alginates
have molecular weights in this range (3-500.000
Da). Undegraded bacterial alginates may have
molecular weights 10x higher.
The sugar rings of M and G are rather inflexible, but
the glycosidic linkages allow some rotation. This
accounts for the ‘random coil’ appearance of long
alginate chains. The macromolecular extension of
alginates are further illustrated by measurements of
the radius of gyration (R
G
) and intrinsic viscosity (
[η]
) for alginates covering a
13
If you have forgot simple acid-base titration theory (which we use a lot in this course) this is the time to read it again.
Figure 21. Electron
micrographs of alginate
(top) and xanthan
(bottom). Scale bar: 200
nm

