Basic HTML Version
20
Alginates are used in the food industry (as thickeners and stabilizers), and in
the pharmaceutical industry (tablet formulations, drug delivery, biomaterials
for tissue engineering etc.) mainly due to their viscosifying and gelling (with
Ca
++
) properties, but are also used in textile printing pastes, surface treatment
of paper and cardboard, in welding electrodes.
Most industrial applications of alginates are based on the ability of alginate
solutions (2-10%) to form gels when calcium salts are added.
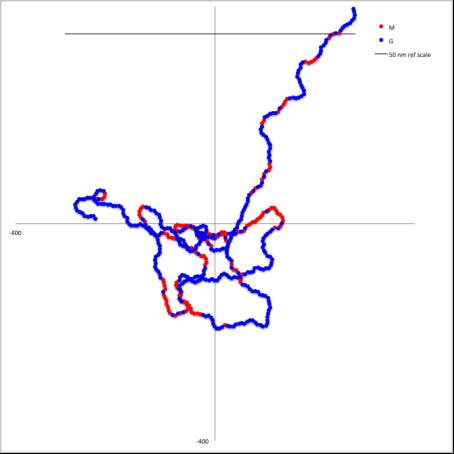
1.2.3. Structure of alginates
Alginates are sometimes described as binary
co-
polymers because they
contain two different monomers (abbreviated M and G), arranged in a variety
of sequences. They are further linear, meaning they are not branched.
Until the biosynthesis of alginates was fully understood (quite recently),
statistical considerations adapted from the science of synthetic copolymers
were used. It was believed alginate chains were co-polymerized from mixtures
of M and G precursors. We now know that alginates are produced
(enzymatically, of course) in a completely different (and even more
interesting) way. Fortunately, this simplifies the understanding of alginate
structures. Another consequence is that the term ‘co-polymer’ is no longer
appropriate
6
.
The first step in the biosynthesis of alginate is to make a homopolymer:
mannuronan (poly-mannuronic acid) from the precursor GDP-D-mannuronic
acid:
6
Copolymers normally refer to polymers made up in a polymerisation process starting from a
mixture of two monomers: nA + mB
→
A
n
B
m
. As we will see, alginates are certainly not made
in this way.

