Basic HTML Version
197
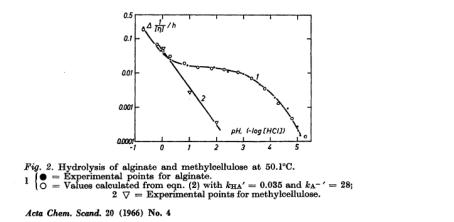
The S-like shape obtained for alginate (but not methyl cellulose) is attributed
to the protonation of the carboxylic groups at C6:
-COO
-
+ H
+
↔
-COOH (pK
a
= ca. 3 for uronic acids)
The C-6 proton can, because of its location, directly protonate the adjacent
glycosidic oxygen.
5.1.5. Side
reactions
in
strong acids
In strong acids reducing sugars become reactive. They can on one hand
eliminate water in a series of reactions leading to furfurals, which are
generally coloured and react easily with other molecules (e.g. amino acids,
phenols..). Also, reducing sugars can initiate reactions with proteins (free
amino groups), initiating the classical browning seen when heating sugars and
amino acids (Maillard products.)
Free sugars also react with dissolved O
2
. Thus, hydrolysis should be carried
out in the absence of oxygen, typically nitrogen or argon atmosphere.
O
OH
HO
OH
O
O
O
OH
HO
O
O
O
H
O
OH
HO
OH
O
O
O
OH
HO
O
O
H
O

