Basic HTML Version
133
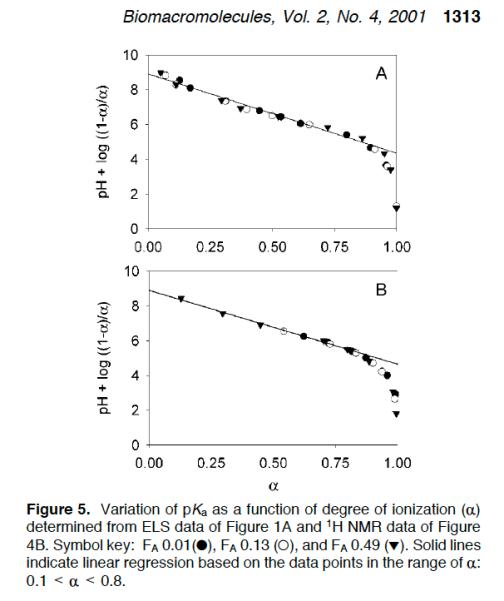
Finally, the two different titration methods provide the same Katchalsky plots.
Note that the parameter
α
stands for the degree of ionization in these figures,
not degree of dissociation.
The plot shows that pK
A
depends on the degree of ionization (‘
α
’), just as for
hyaluronan, and for the same reason, except that pK
a
decreases with
increasing ionization, opposite of polyanions. As charges are removed
(extrapolation to zero charge), pK
a
approaches 8.8-8.9, which is also pK
a
of
the monomer.
3.1.11. Polyelectrolyte
complexes
Polyelectrolyte complexes form when oppositely charged polymers are mixed.
Such complexes may take many forms, e.g. insoluble fibres, gels, dispersed
nanoparticles. Examples include DNA-chitosan complexes, which can be
used as gene delivery vehicles. However, to illustrate the basic principles and
how we can use pH to control the complexes, the alginate-chitosan system
may be used.
Alginate:
The carboxyl group has a pK
A
of ca. 3.5. The charge density for a carboxyl
group is -
α
, i.e. one negative charge (-COO
-
) per dissociated -COOH. It
follows the alginate is 10% charged (
α
= -0.10) at pH 2.5, 50% charged (
α
=

